Key Insights
The European Minimally Invasive Surgery (MIS) devices market is experiencing robust growth, driven by several key factors. The increasing prevalence of chronic diseases like cardiovascular and gastrointestinal disorders, coupled with a rising geriatric population requiring more surgical interventions, fuels market expansion. Technological advancements in robotic surgery, image-guided systems, and minimally invasive surgical tools are enhancing surgical precision, reducing recovery times, and improving patient outcomes, thereby boosting market adoption. Furthermore, a preference for less invasive procedures among both patients and surgeons, along with supportive government initiatives promoting advanced medical technologies, contribute significantly to market growth. The market is segmented by application (cardiovascular, gastrointestinal, gynecological, orthopedic, urological, and others) and product type (handheld instruments, guiding devices, electrosurgical devices, endoscopic devices, laparoscopic devices, monitoring and visualization devices, robotic-assisted surgical systems, ablation devices, laser-based devices, and others). Major players like Smith & Nephew, GE Healthcare, Philips, Intuitive Surgical, and Medtronic are actively driving innovation and expanding their market presence through strategic collaborations and product launches.
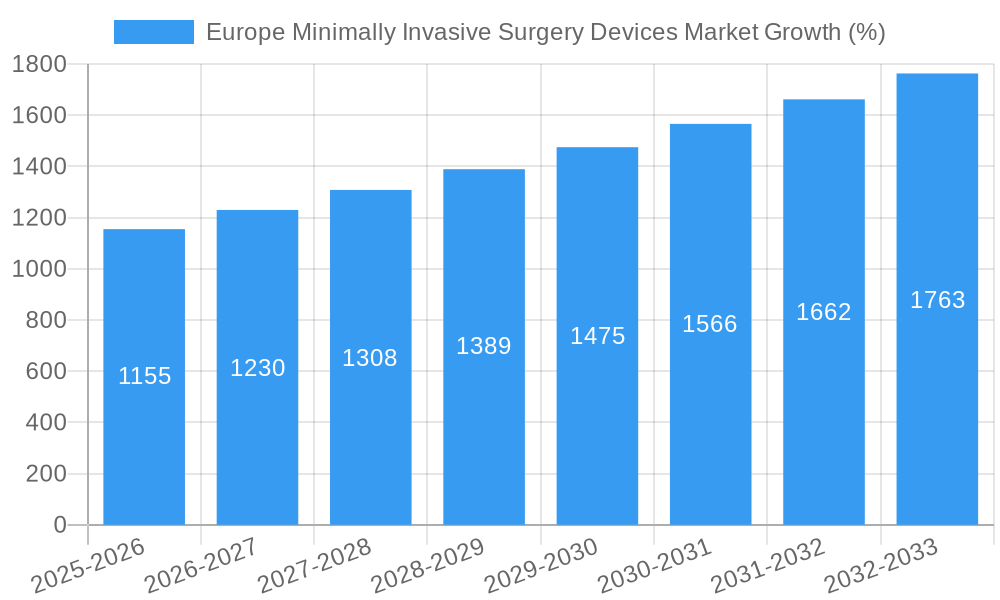
Within the European region, Germany, France, the United Kingdom, and Italy represent significant market segments, reflecting their advanced healthcare infrastructure and high adoption rates of MIS procedures. The CAGR of 7.70% indicates a consistent upward trajectory, with projected market expansion throughout the forecast period (2025-2033). While potential restraints, such as high costs associated with advanced MIS devices and stringent regulatory approvals, might somewhat hinder growth, the overall market outlook remains highly positive due to the overarching trends favoring minimally invasive surgical techniques and the continuous evolution of the technological landscape. The consistent rise in demand for less invasive, faster-recovery surgery will continue to drive market growth, making this a lucrative sector for investment and innovation.

Europe Minimally Invasive Surgery Devices Market: A Comprehensive Report (2019-2033)
This in-depth report provides a comprehensive analysis of the Europe Minimally Invasive Surgery (MIS) Devices market, offering valuable insights for industry professionals, investors, and strategic decision-makers. Covering the period from 2019 to 2033, with a base year of 2025 and a forecast period of 2025-2033, this report unveils the market's structure, dynamics, and future trajectory. The market size is projected to reach xx Million by 2033, exhibiting a robust CAGR of xx% during the forecast period.
Europe Minimally Invasive Surgery Devices Market Market Structure & Innovation Trends
The European MIS devices market exhibits a moderately consolidated structure, with key players like Smith & Nephew, General Electric Company (GE Healthcare), Koninklijke Philips NV, Intuitive Surgical Inc, Siemens Healthineers, Medtronic, Stryker Corporation, Zimmer Biomet, Abbott Laboratories, and Olympus Corporation holding significant market share. The exact market share distribution varies across segments but is generally characterized by intense competition. Innovation is a crucial driver, fueled by advancements in robotics, imaging, and materials science. Stringent regulatory frameworks, such as those established by the European Medicines Agency (EMA), significantly influence product development and market entry. The market also sees ongoing mergers and acquisitions (M&A) activity, with deal values reaching xx Million in recent years. These activities are often driven by efforts to expand product portfolios, gain access to new technologies, and increase market reach. Substitute products, such as traditional open surgery methods, continue to exist, but the growth of MIS is primarily driven by its benefits including reduced patient trauma, faster recovery times, and shorter hospital stays. End-user demographics are shifting with an aging population and increased prevalence of chronic diseases contributing to rising demand.
- Market Concentration: Moderately consolidated
- Innovation Drivers: Robotics, Imaging, Materials Science
- Regulatory Framework: Stringent (EMA)
- M&A Activity: Significant, with deal values reaching xx Million annually.
- Product Substitutes: Traditional open surgery
- End-User Demographics: Aging population, increasing chronic disease prevalence
Europe Minimally Invasive Surgery Devices Market Market Dynamics & Trends
The European MIS devices market is experiencing robust growth driven by several factors. Increasing prevalence of chronic diseases, a preference for minimally invasive procedures among both patients and surgeons, technological advancements resulting in enhanced precision and efficacy, and favorable reimbursement policies are all key drivers. Technological disruptions, such as the introduction of robotic-assisted surgical systems and advanced imaging techniques, are reshaping the market landscape. Consumer preferences are shifting towards less invasive procedures with faster recovery times, fueling demand for sophisticated MIS devices. Competitive dynamics are intense, with major players focusing on R&D, strategic partnerships, and acquisitions to maintain their market position. Market penetration for robotic-assisted surgery is growing steadily, expected to reach xx% by 2033. The overall market exhibits a high growth potential, largely influenced by the aforementioned trends. The overall market is expected to grow at a CAGR of xx% during the forecast period, driven primarily by technological advancements and increasing preference for minimally invasive procedures.

Dominant Regions & Segments in Europe Minimally Invasive Surgery Devices Market
Within Europe, Germany, France, and the UK represent the leading markets for MIS devices. This dominance stems from factors such as well-established healthcare infrastructure, high healthcare expenditure, and a significant concentration of specialized medical centers.
Key Drivers for Dominant Regions:
- Germany, France, UK: Advanced healthcare infrastructure, high healthcare expenditure, presence of specialized medical centers.
Dominant Segments:
- By Application: Cardiovascular and Orthopedic segments are projected to hold the largest market share due to the high prevalence of associated diseases and the increasing adoption of minimally invasive techniques.
- By Product: Robotic-assisted surgical systems and endoscopic devices are expected to witness significant growth due to their enhanced capabilities and growing demand for improved surgical outcomes. The high cost of robotic systems compared to traditional laparoscopic devices leads to uneven market penetration across different countries within the region.
Europe Minimally Invasive Surgery Devices Market Product Innovations
Recent product innovations in the European MIS devices market include advanced robotic systems offering enhanced dexterity and precision, improved imaging technologies for better visualization during procedures, and the development of minimally invasive instruments tailored for specific surgical applications. These innovations improve surgical outcomes, reduce complication rates, and enhance patient recovery. Companies are focusing on developing minimally invasive devices with greater functionality while enhancing their safety and efficiency. The market is witnessing a shift towards smart devices with better integration capabilities allowing surgeons to optimize their workflow.
Report Scope & Segmentation Analysis
This report offers a comprehensive segmentation of the Europe Minimally Invasive Surgery (MIS) devices market, categorized by application (Cardiovascular, Gastrointestinal, Gynecological, Orthopedic, Urological, and Other Applications) and product type (Handheld Instruments, Guiding Devices, Electrosurgical Devices, Endoscopic Devices, Laparoscopic Devices, Monitoring and Visualization Devices, Robotic-Assisted Surgical Systems, Ablation Devices, Laser-Based Devices, and Other MIS Devices). A detailed analysis of each segment is provided, encompassing market size, growth rate, and competitive dynamics. Furthermore, the report presents growth projections for each segment, highlighting potential for future expansion and lucrative investment opportunities. This granular breakdown allows for a precise understanding of market trends and their implications for stakeholders.
Key Drivers of Europe Minimally Invasive Surgery Devices Market Growth
The growth of the European MIS devices market is propelled by several factors: an aging population leading to an increased incidence of chronic diseases requiring surgical intervention, technological advancements that enhance surgical precision and reduce recovery times, favorable reimbursement policies that support the adoption of MIS procedures, and a growing preference among surgeons and patients for minimally invasive techniques due to their associated benefits. Furthermore, increasing healthcare expenditure and improving healthcare infrastructure in several European countries contribute to market growth.
Challenges in the Europe Minimally Invasive Surgery Devices Market Sector
The European MIS devices market faces challenges including the high cost of advanced devices, stringent regulatory requirements for market access, potential supply chain disruptions affecting the availability of components, and intense competition among established players and new entrants. These factors can impact market expansion and profitability. The reimbursement landscape also poses a challenge, particularly for high-cost devices like robotic systems. Furthermore, variations in healthcare regulations across different European countries create complexity for companies operating across the region.
Emerging Opportunities in Europe Minimally Invasive Surgery Devices Market
The European MIS devices market presents several compelling emerging opportunities. Significant growth potential lies in the development and adoption of innovative technologies such as AI-powered surgical assistants, which promise enhanced precision and efficiency. The rise of personalized surgical solutions, tailored to individual patient data and needs, is another key driver. Improved integration of various MIS devices within a single surgical workflow aims to streamline procedures and optimize outcomes. Expanding minimally invasive procedures into new therapeutic areas and geographical regions within Europe also offers considerable growth potential. Companies that effectively address the unique healthcare needs of the European market and adapt their offerings to evolving regulatory landscapes and patient preferences are best positioned to capitalize on these opportunities.
Leading Players in the Europe Minimally Invasive Surgery Devices Market Market
- Smith & Nephew
- General Electric Company (GE Healthcare)
- Koninklijke Philips NV
- Intuitive Surgical Inc
- Siemens Healthineers
- Medtronic
- Stryker Corporation
- Zimmer Biomet
- Abbott Laboratories
- Olympus Corporation
Key Developments in Europe Minimally Invasive Surgery Devices Industry
- February 2022: Medtronic plc and OLV Hospital Aalst announced the first European clinical procedure using the Hugo robotic-assisted surgery (RAS) system, marking a significant step in expanding RAS adoption within Europe.
- March 2022: Quantum Surgical received FDA 510(k) clearance for Epione, a novel interventional oncology robotics system for minimally invasive liver cancer treatment. While not specifically European regulatory approval, this indicates significant technological advancement with potential future impact on the European market.
Future Outlook for Europe Minimally Invasive Surgery Devices Market Market
The future of the European MIS devices market appears promising, driven by continuous technological advancements, increasing adoption of minimally invasive procedures, and a growing focus on improving patient outcomes. Strategic partnerships, acquisitions, and expansion into new therapeutic areas will shape the market's competitive landscape. The market's growth trajectory is expected to be robust, fueled by these positive trends. Companies that successfully adapt to evolving regulatory frameworks and consumer preferences will likely secure strong market positions in the years ahead.
Europe Minimally Invasive Surgery Devices Market Segmentation
-
1. Products
- 1.1. Handheld Instruments
- 1.2. Guiding Devices
- 1.3. Electrosurgical Devices
- 1.4. Endoscopic Devices
- 1.5. Laproscopic Devices
- 1.6. Monitoring and Visualization Devices
- 1.7. Robotic Assisted Surgical Systems
- 1.8. Ablation Devices
- 1.9. Laser Based Devices
- 1.10. Other MIS Devices
-
2. Application
- 2.1. Cardiovascular
- 2.2. Gastrointestinal
- 2.3. Gynecological
- 2.4. Orthopedic
- 2.5. Urological
- 2.6. Other Applications
- 3. Geography
- 4. Germany
- 5. United Kingdom
- 6. France
- 7. Italy
- 8. Spain
- 9. Rest of Europe
Europe Minimally Invasive Surgery Devices Market Segmentation By Geography
-
1. Europe
- 1.1. United Kingdom
- 1.2. Germany
- 1.3. France
- 1.4. Italy
- 1.5. Spain
- 1.6. Netherlands
- 1.7. Belgium
- 1.8. Sweden
- 1.9. Norway
- 1.10. Poland
- 1.11. Denmark

Europe Minimally Invasive Surgery Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 7.70% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence of Lifestyle-related and Chronic Disorders; Higher Acceptance Rate of Minimally Invasive Surgeries Over Traditional Surgeries; Technological Advancements in Minimally Invasive Devices
- 3.3. Market Restrains
- 3.3.1. Shortage of Experienced Professionals
- 3.4. Market Trends
- 3.4.1. Gastrointestinal Segment is Expected to be the Fastest Growing Segment Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Products
- 5.1.1. Handheld Instruments
- 5.1.2. Guiding Devices
- 5.1.3. Electrosurgical Devices
- 5.1.4. Endoscopic Devices
- 5.1.5. Laproscopic Devices
- 5.1.6. Monitoring and Visualization Devices
- 5.1.7. Robotic Assisted Surgical Systems
- 5.1.8. Ablation Devices
- 5.1.9. Laser Based Devices
- 5.1.10. Other MIS Devices
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Cardiovascular
- 5.2.2. Gastrointestinal
- 5.2.3. Gynecological
- 5.2.4. Orthopedic
- 5.2.5. Urological
- 5.2.6. Other Applications
- 5.3. Market Analysis, Insights and Forecast - by Geography
- 5.4. Market Analysis, Insights and Forecast - by Germany
- 5.5. Market Analysis, Insights and Forecast - by United Kingdom
- 5.6. Market Analysis, Insights and Forecast - by France
- 5.7. Market Analysis, Insights and Forecast - by Italy
- 5.8. Market Analysis, Insights and Forecast - by Spain
- 5.9. Market Analysis, Insights and Forecast - by Rest of Europe
- 5.10. Market Analysis, Insights and Forecast - by Region
- 5.10.1. Europe
- 5.1. Market Analysis, Insights and Forecast - by Products
- 6. Germany Europe Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 7. France Europe Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 8. Italy Europe Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 9. United Kingdom Europe Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 10. Netherlands Europe Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 11. Sweden Europe Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 12. Rest of Europe Europe Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 13. Competitive Analysis
- 13.1. Market Share Analysis 2024
- 13.2. Company Profiles
- 13.2.1 Smith & Nephew
- 13.2.1.1. Overview
- 13.2.1.2. Products
- 13.2.1.3. SWOT Analysis
- 13.2.1.4. Recent Developments
- 13.2.1.5. Financials (Based on Availability)
- 13.2.2 General Electric Company (GE Healthcare)
- 13.2.2.1. Overview
- 13.2.2.2. Products
- 13.2.2.3. SWOT Analysis
- 13.2.2.4. Recent Developments
- 13.2.2.5. Financials (Based on Availability)
- 13.2.3 Koninklijke Philips NV
- 13.2.3.1. Overview
- 13.2.3.2. Products
- 13.2.3.3. SWOT Analysis
- 13.2.3.4. Recent Developments
- 13.2.3.5. Financials (Based on Availability)
- 13.2.4 Intuitive Surgical Inc
- 13.2.4.1. Overview
- 13.2.4.2. Products
- 13.2.4.3. SWOT Analysis
- 13.2.4.4. Recent Developments
- 13.2.4.5. Financials (Based on Availability)
- 13.2.5 Siemens Healthineers
- 13.2.5.1. Overview
- 13.2.5.2. Products
- 13.2.5.3. SWOT Analysis
- 13.2.5.4. Recent Developments
- 13.2.5.5. Financials (Based on Availability)
- 13.2.6 Medtronic
- 13.2.6.1. Overview
- 13.2.6.2. Products
- 13.2.6.3. SWOT Analysis
- 13.2.6.4. Recent Developments
- 13.2.6.5. Financials (Based on Availability)
- 13.2.7 Stryker Corporation
- 13.2.7.1. Overview
- 13.2.7.2. Products
- 13.2.7.3. SWOT Analysis
- 13.2.7.4. Recent Developments
- 13.2.7.5. Financials (Based on Availability)
- 13.2.8 Zimmer Biomet
- 13.2.8.1. Overview
- 13.2.8.2. Products
- 13.2.8.3. SWOT Analysis
- 13.2.8.4. Recent Developments
- 13.2.8.5. Financials (Based on Availability)
- 13.2.9 Abbott Laboratories
- 13.2.9.1. Overview
- 13.2.9.2. Products
- 13.2.9.3. SWOT Analysis
- 13.2.9.4. Recent Developments
- 13.2.9.5. Financials (Based on Availability)
- 13.2.10 Olympus Corporation
- 13.2.10.1. Overview
- 13.2.10.2. Products
- 13.2.10.3. SWOT Analysis
- 13.2.10.4. Recent Developments
- 13.2.10.5. Financials (Based on Availability)
- 13.2.1 Smith & Nephew
List of Figures
- Figure 1: Europe Minimally Invasive Surgery Devices Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Minimally Invasive Surgery Devices Market Share (%) by Company 2024
List of Tables
- Table 1: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 3: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Products 2019 & 2032
- Table 4: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Products 2019 & 2032
- Table 5: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Application 2019 & 2032
- Table 6: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Application 2019 & 2032
- Table 7: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Geography 2019 & 2032
- Table 8: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Geography 2019 & 2032
- Table 9: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Germany 2019 & 2032
- Table 10: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Germany 2019 & 2032
- Table 11: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by United Kingdom 2019 & 2032
- Table 12: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by United Kingdom 2019 & 2032
- Table 13: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by France 2019 & 2032
- Table 14: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by France 2019 & 2032
- Table 15: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Italy 2019 & 2032
- Table 16: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Italy 2019 & 2032
- Table 17: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Spain 2019 & 2032
- Table 18: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Spain 2019 & 2032
- Table 19: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Rest of Europe 2019 & 2032
- Table 20: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Rest of Europe 2019 & 2032
- Table 21: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 22: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 23: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 24: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 25: Germany Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 26: Germany Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 27: France Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 28: France Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 29: Italy Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 30: Italy Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 31: United Kingdom Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 32: United Kingdom Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 33: Netherlands Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 34: Netherlands Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 35: Sweden Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 36: Sweden Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 37: Rest of Europe Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 38: Rest of Europe Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 39: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Products 2019 & 2032
- Table 40: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Products 2019 & 2032
- Table 41: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Application 2019 & 2032
- Table 42: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Application 2019 & 2032
- Table 43: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Geography 2019 & 2032
- Table 44: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Geography 2019 & 2032
- Table 45: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Germany 2019 & 2032
- Table 46: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Germany 2019 & 2032
- Table 47: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by United Kingdom 2019 & 2032
- Table 48: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by United Kingdom 2019 & 2032
- Table 49: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by France 2019 & 2032
- Table 50: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by France 2019 & 2032
- Table 51: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Italy 2019 & 2032
- Table 52: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Italy 2019 & 2032
- Table 53: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Spain 2019 & 2032
- Table 54: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Spain 2019 & 2032
- Table 55: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Rest of Europe 2019 & 2032
- Table 56: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Rest of Europe 2019 & 2032
- Table 57: Europe Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 58: Europe Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 59: United Kingdom Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 60: United Kingdom Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 61: Germany Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 62: Germany Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 63: France Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 64: France Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 65: Italy Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 66: Italy Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 67: Spain Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 68: Spain Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 69: Netherlands Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 70: Netherlands Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 71: Belgium Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 72: Belgium Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 73: Sweden Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 74: Sweden Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 75: Norway Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 76: Norway Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 77: Poland Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 78: Poland Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 79: Denmark Europe Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 80: Denmark Europe Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Minimally Invasive Surgery Devices Market?
The projected CAGR is approximately 7.70%.
2. Which companies are prominent players in the Europe Minimally Invasive Surgery Devices Market?
Key companies in the market include Smith & Nephew, General Electric Company (GE Healthcare), Koninklijke Philips NV, Intuitive Surgical Inc, Siemens Healthineers, Medtronic, Stryker Corporation, Zimmer Biomet, Abbott Laboratories, Olympus Corporation.
3. What are the main segments of the Europe Minimally Invasive Surgery Devices Market?
The market segments include Products, Application, Geography, Germany, United Kingdom, France, Italy, Spain, Rest of Europe.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of Lifestyle-related and Chronic Disorders; Higher Acceptance Rate of Minimally Invasive Surgeries Over Traditional Surgeries; Technological Advancements in Minimally Invasive Devices.
6. What are the notable trends driving market growth?
Gastrointestinal Segment is Expected to be the Fastest Growing Segment Over the Forecast Period.
7. Are there any restraints impacting market growth?
Shortage of Experienced Professionals.
8. Can you provide examples of recent developments in the market?
February 2022: Medtronic plc and OLV Hospital Aalst announced that the first clinical procedure in Europe had been performed with the Hugo robotic-assisted surgery (RAS) system.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Minimally Invasive Surgery Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Minimally Invasive Surgery Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Minimally Invasive Surgery Devices Market?
To stay informed about further developments, trends, and reports in the Europe Minimally Invasive Surgery Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



