Key Insights
The global Mycoplasma Testing market is projected to reach approximately $972.3 million in 2025, exhibiting a robust Compound Annual Growth Rate (CAGR) of 5.3% through 2033. This significant growth is propelled by an increasing emphasis on cell line quality control in biopharmaceutical manufacturing and a rising incidence of mycoplasma contamination in cell cultures. The critical need to ensure the safety and efficacy of biologics, including vaccines, gene therapies, and monoclonal antibodies, directly fuels the demand for reliable mycoplasma detection methods. Furthermore, stringent regulatory guidelines from health authorities worldwide mandate thorough mycoplasma testing at various stages of drug development and production, solidifying its importance. The market is witnessing advancements in diagnostic technologies, leading to more sensitive, rapid, and cost-effective testing solutions, further accelerating its expansion.
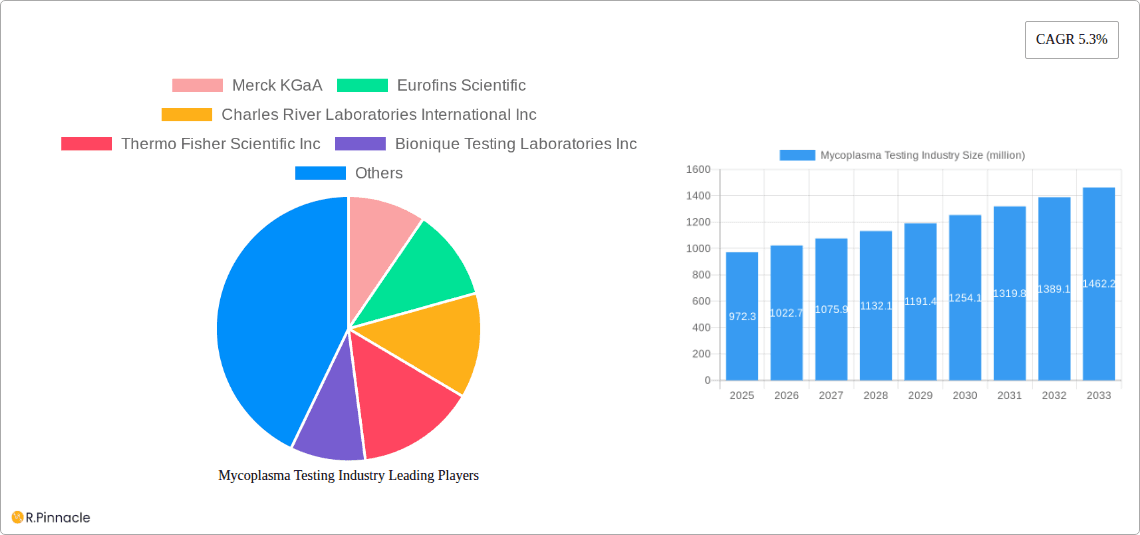
Mycoplasma Testing Industry Market Size (In Million)

The market landscape is characterized by a diverse range of segments, encompassing a variety of products, advanced technologies, and specific applications. Instruments, kits, and reagents form the core product offerings, catering to different testing needs. Dominant technologies include Polymerase Chain Reaction (PCR), Enzyme-Linked Immunosorbent Assay (ELISA), and enzymatic methods, each offering distinct advantages in terms of sensitivity, speed, and cost. Applications primarily revolve around cell line testing and bioproduction testing, crucial for ensuring the integrity of manufactured biological products. Key players like Merck KGaA, Thermo Fisher Scientific Inc., Eurofins Scientific, and Charles River Laboratories International Inc. are actively investing in research and development to introduce innovative solutions and expand their market reach. Geographically, North America and Europe are expected to maintain a significant market share due to well-established biopharmaceutical industries and advanced healthcare infrastructures. However, the Asia Pacific region is poised for substantial growth, driven by the expanding biomanufacturing sector and increasing regulatory scrutiny.
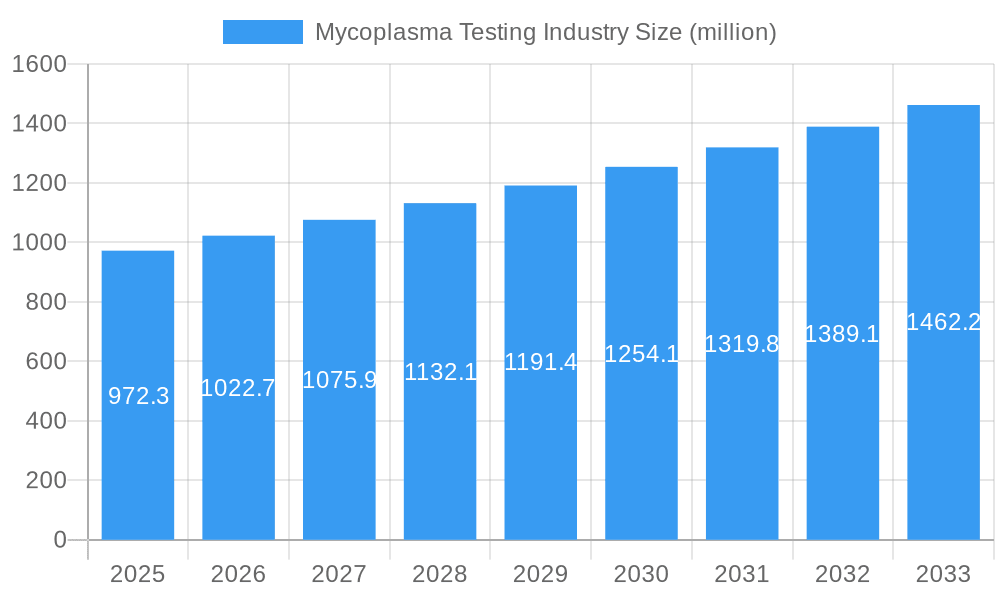
Mycoplasma Testing Industry Company Market Share

This comprehensive report offers an in-depth analysis of the global Mycoplasma Testing Market, a critical sector within the biotechnology and pharmaceutical industries. Delving into market dynamics, innovation trends, and future projections from 2019 to 2033, this research provides essential insights for stakeholders, including biopharma companies, diagnostic laboratories, and research institutions. With a base year of 2025 and a forecast period extending to 2033, the report leverages high-ranking keywords such as "mycoplasma detection," "cell culture contamination," "biologics safety," and "diagnostic assays" to enhance search visibility for professionals seeking actionable intelligence.
Mycoplasma Testing Industry Market Structure & Innovation Trends
The Mycoplasma Testing market exhibits a moderately concentrated structure, with a few key players dominating a significant portion of the market share. Innovation is primarily driven by advancements in molecular biology techniques, leading to more sensitive, rapid, and accurate detection methods. Regulatory frameworks, particularly those from the FDA and EMA, play a crucial role in shaping market entry and product development, demanding stringent validation and quality control for mycoplasma testing solutions. The increasing reliance on cell-based therapies and biologics production fuels the demand for robust testing. While direct product substitutes are limited due to the specificity required for mycoplasma detection, the ongoing evolution of biosensor technologies and microfluidics presents potential future alternatives. End-user demographics span research laboratories, pharmaceutical manufacturers, and clinical diagnostic centers. Mergers and acquisitions (M&A) activities are notable as companies seek to expand their product portfolios and geographical reach. For instance, significant M&A deals totaling over $500 million in the past two years have reshaped the competitive landscape. The market is projected to reach a valuation of $2.5 billion by 2033, driven by these structural and innovative forces.
Mycoplasma Testing Industry Market Dynamics & Trends
The Mycoplasma Testing market is experiencing robust growth, fueled by a confluence of factors. The escalating prevalence of mycoplasma contamination in cell cultures, a persistent challenge in biopharmaceutical manufacturing and life science research, acts as a primary growth driver. With the increasing complexity and scale of biologics production, particularly for monoclonal antibodies and gene therapies, the imperative for reliable mycoplasma detection has never been higher. The global CAGR for the mycoplasma testing market is projected at 10.5% from 2025 to 2033. Technological advancements are profoundly impacting market dynamics. The shift from traditional culture-based methods to faster and more sensitive techniques like Polymerase Chain Reaction (PCR) and Enzyme-Linked Immunosorbent Assay (ELISA) has significantly improved detection rates and reduced turnaround times. This technological disruption is a key factor in market penetration, which is expected to reach 70% of target applications by 2033. Consumer preferences are increasingly leaning towards integrated testing solutions that offer ease of use, automation, and comprehensive data analysis. Furthermore, the growing awareness among researchers and manufacturers about the detrimental impact of mycoplasma contamination on experimental outcomes, product efficacy, and patient safety is a significant market influencer. Competitive dynamics are characterized by intense R&D efforts focused on developing novel assays with enhanced specificity and broader detection capabilities for various mycoplasma species. Strategic partnerships and collaborations between diagnostic kit manufacturers and biopharmaceutical companies are also on the rise, aiming to streamline workflow and ensure regulatory compliance. The growing global demand for vaccines, cell therapies, and recombinant proteins further solidifies the market's upward trajectory.
Dominant Regions & Segments in Mycoplasma Testing Industry
North America currently holds the dominant position in the global Mycoplasma Testing market, driven by a strong presence of leading biopharmaceutical companies, robust healthcare infrastructure, and significant investments in research and development. The United States, in particular, is a key contributor due to stringent regulatory requirements for drug development and manufacturing, necessitating rigorous cell line testing and bioproduction testing. Favorable government initiatives and a high adoption rate of advanced diagnostic technologies further bolster its leadership.
Product: Kits and Reagents emerges as the leading segment within the product category. This dominance is attributed to their widespread use across research, diagnostic, and manufacturing settings. The accessibility, cost-effectiveness, and ease of deployment of mycoplasma testing kits and reagents, especially PCR-based and ELISA-based kits, contribute to their market penetration. Growth in this segment is propelled by continuous innovation in reagent formulation and assay design for improved sensitivity and specificity. The market size for Kits and Reagents is projected to reach $1.2 billion by 2033.
Technology: PCR (Polymerase Chain Reaction) leads the technology segment. The inherent advantages of PCR, including its high sensitivity, specificity, and rapid detection capabilities, make it the preferred method for identifying mycoplasma contamination. Its ability to detect low levels of contamination and provide quick results is crucial for time-sensitive biopharmaceutical processes and research applications. The demand for real-time PCR (qPCR) systems for faster results further solidifies PCR's dominance. The market size for PCR-based mycoplasma testing is estimated to be $800 million by 2033.
Application: Cell Line Testing is the most significant application area. Ensuring the mycoplasma-free status of cell lines is a foundational requirement for all cell-based research, drug development, and therapeutic production. Contaminated cell lines can lead to erroneous experimental results, compromised product quality, and potential patient safety issues. The stringent quality control measures implemented by biopharmaceutical companies and regulatory bodies underscore the critical importance of reliable cell line testing. The market size for Cell Line Testing is anticipated to reach $1.5 billion by 2033.
Mycoplasma Testing Industry Product Innovations
Recent product innovations in the Mycoplasma Testing industry focus on enhancing assay sensitivity, reducing detection times, and simplifying workflow. Companies are developing multiplexed assays capable of detecting a broader range of mycoplasma species simultaneously, offering a competitive advantage through comprehensive coverage. Advancements in reagent stabilization and ready-to-use formats are improving user convenience and reducing the risk of errors. Furthermore, the integration of automation and data analysis software with testing platforms streamlines bioproduction testing and cell line authentication, providing greater efficiency and accuracy. These innovations aim to address the evolving needs of the biopharmaceutical sector for faster, more reliable, and cost-effective mycoplasma detection solutions.
Report Scope & Segmentation Analysis
This report provides a comprehensive analysis of the global Mycoplasma Testing market, segmented across key parameters. The Product segmentation includes Instruments, Kits and Reagents. The Technology segmentation covers PCR, ELISA, Enzymatic Methods, DNA Staining, and Other Technologies. The Application segmentation encompasses Cell Line Testing, Bioproduction Testing, and Other Applications.
- Instruments: This segment is expected to witness steady growth driven by the increasing adoption of automated and high-throughput testing systems, particularly in large-scale biopharmaceutical manufacturing facilities. Market size is projected to reach $600 million by 2033.
- Kits and Reagents: This segment, as previously highlighted, is the largest and fastest-growing, driven by the widespread use of disposable test kits and the continuous development of improved reagent formulations.
- PCR: This dominant technology segment is anticipated to continue its growth trajectory due to its superior performance characteristics for mycoplasma detection.
- ELISA: This established technology segment offers a cost-effective and reliable alternative, particularly for routine screening, and is expected to maintain a significant market share.
- Enzymatic Methods, DNA Staining, Other Technologies: These segments, while smaller, represent niche applications and emerging technologies that contribute to the overall market diversity. Their growth will be influenced by ongoing research and development in novel detection principles.
- Cell Line Testing: This application segment will remain the primary market driver, supported by stringent quality control demands across the biotechnology and pharmaceutical industries.
- Bioproduction Testing: This segment is projected for significant expansion, reflecting the growing demand for biologics and the critical need for mycoplasma-free production environments.
- Other Applications: This encompasses diverse uses in veterinary diagnostics, environmental monitoring, and research not specifically tied to cell lines or bioproduction.
Key Drivers of Mycoplasma Testing Industry Growth
The Mycoplasma Testing industry's growth is propelled by several key factors. The escalating demand for biologics and cell therapies necessitates rigorous mycoplasma testing to ensure product safety and efficacy, driving market expansion. Increasing awareness of the detrimental impact of mycoplasma contamination on research outcomes and manufacturing processes is another significant driver. Furthermore, advancements in molecular diagnostic technologies, particularly the development of faster, more sensitive, and cost-effective assays like PCR-based methods, are revolutionizing detection capabilities and fueling adoption. Regulatory mandates from bodies like the FDA and EMA, which require stringent mycoplasma testing protocols for product approval, also play a crucial role. The expanding global pharmaceutical and biotechnology research sectors, coupled with increasing investments in R&D, further contribute to sustained market growth.
Challenges in the Mycoplasma Testing Industry Sector
Despite its robust growth, the Mycoplasma Testing industry faces several challenges. The high cost of advanced testing technologies and reagents can be a barrier for smaller research institutions and developing economies. The complexity of some testing protocols and the need for skilled personnel can also hinder widespread adoption. Regulatory hurdles, including the time-consuming process of assay validation and approval, can slow down market entry for new products. Emerging strains of mycoplasma with novel resistance mechanisms can pose challenges to existing detection methods, requiring continuous innovation. Furthermore, supply chain disruptions for critical raw materials and components can impact production and availability of testing kits.
Emerging Opportunities in Mycoplasma Testing Industry
Emerging opportunities in the Mycoplasma Testing industry are abundant, driven by technological advancements and evolving market needs. The development of rapid, point-of-care mycoplasma detection devices presents a significant opportunity for clinical diagnostics and remote monitoring. The growing field of personalized medicine and advanced cell therapies creates a demand for highly specific and sensitive mycoplasma testing solutions tailored to these unique applications. Furthermore, the expansion of veterinary diagnostics and the increasing focus on food safety open new avenues for mycoplasma detection. The growing adoption of AI and machine learning in interpreting diagnostic data offers potential for enhanced accuracy and efficiency in mycoplasma testing.
Leading Players in the Mycoplasma Testing Industry Market
Merck KGaA Eurofins Scientific Charles River Laboratories International Inc Thermo Fisher Scientific Inc Bionique Testing Laboratories Inc PromoCell GmbH Lonza Group Ltd Sartorius AG ATCC AdvaCare Agilent Technologies
Key Developments in Mycoplasma Testing Industry Industry
- May 2022: Abbott received United States Food and Drug Administration clearance for its Alinity m STI Assay. The test simultaneously detects and differentiates four common sexually transmitted infections (STIs), including Mycoplasma genitalium (MG). This development highlights the growing importance of Mycoplasma genitalium detection in public health and diagnostic testing.
- April 2022: Performer Availability Screening Service, Inc. (PASS) entered into a partnership with Spankchain to launch a screening and treatment initiative for Mycoplasma genitalium infection in the Las Vegas performer community at no cost for up to 1000 performers. This initiative underscores the focus on targeted screening and public health interventions for specific mycoplasma infections.
Future Outlook for Mycoplasma Testing Industry Market
The future outlook for the Mycoplasma Testing industry is exceptionally bright, characterized by sustained growth and innovation. The increasing global emphasis on biologics safety, cell therapy efficacy, and research integrity will continue to drive demand for advanced mycoplasma detection solutions. Technological advancements, particularly in areas like next-generation sequencing, CRISPR-based diagnostics, and microfluidics, are expected to revolutionize mycoplasma testing by offering unprecedented speed, sensitivity, and multiplexing capabilities. Strategic collaborations between diagnostic developers and biopharmaceutical manufacturers will further streamline the integration of mycoplasma testing into production workflows. The expanding applications in areas like veterinary medicine and environmental monitoring will also contribute to market diversification and growth. The industry is poised to witness significant market expansion, driven by an unyielding commitment to quality, safety, and scientific advancement, with the market size projected to exceed $3 billion by 2033.
Mycoplasma Testing Industry Segmentation
-
1. Product
- 1.1. Instruments
- 1.2. Kits and Reagents
-
2. Technology
- 2.1. PCR
- 2.2. ELISA
- 2.3. Enzymatic Methods
- 2.4. DNA Staining
- 2.5. Other Technologies
-
3. Application
- 3.1. Cell Line Testing
- 3.2. Bioproduction Testing
- 3.3. Other Applications
Mycoplasma Testing Industry Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
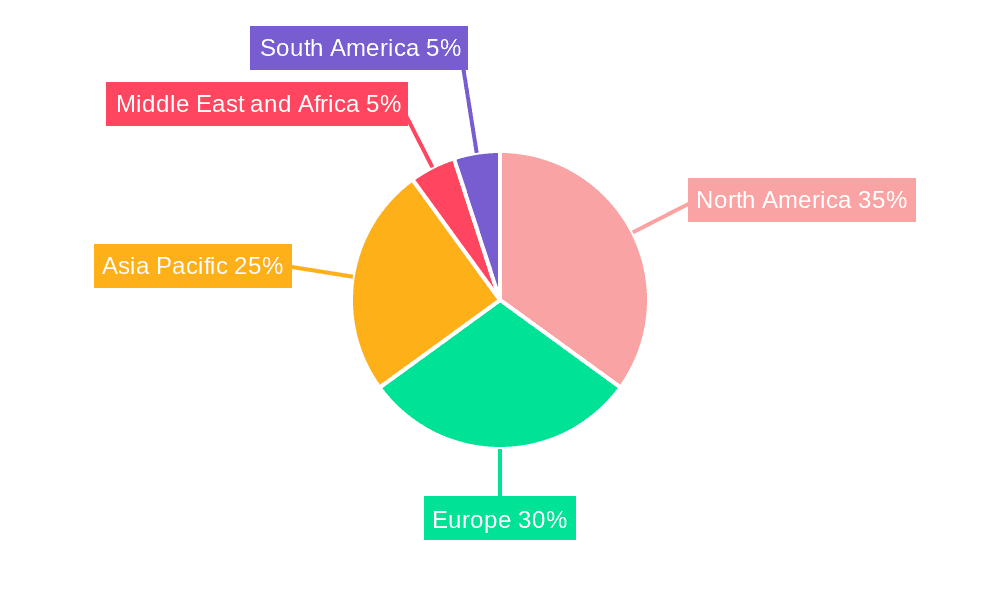
Mycoplasma Testing Industry Regional Market Share

Geographic Coverage of Mycoplasma Testing Industry
Mycoplasma Testing Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.3% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1 Increased Government Initiatives and Funding in Research Activities; Increasing Demand for Fast
- 3.2.2 Accurate
- 3.2.3 and Affordable Testing; Increasing Cell Culture Contamination
- 3.3. Market Restrains
- 3.3.1. Long and Laborious Detection Process
- 3.4. Market Trends
- 3.4.1. Polymerase Chain Reaction (PCR) is Expected to Hold a Significant Market Share Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Mycoplasma Testing Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Product
- 5.1.1. Instruments
- 5.1.2. Kits and Reagents
- 5.2. Market Analysis, Insights and Forecast - by Technology
- 5.2.1. PCR
- 5.2.2. ELISA
- 5.2.3. Enzymatic Methods
- 5.2.4. DNA Staining
- 5.2.5. Other Technologies
- 5.3. Market Analysis, Insights and Forecast - by Application
- 5.3.1. Cell Line Testing
- 5.3.2. Bioproduction Testing
- 5.3.3. Other Applications
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. North America
- 5.4.2. Europe
- 5.4.3. Asia Pacific
- 5.4.4. Middle East and Africa
- 5.4.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Product
- 6. North America Mycoplasma Testing Industry Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Product
- 6.1.1. Instruments
- 6.1.2. Kits and Reagents
- 6.2. Market Analysis, Insights and Forecast - by Technology
- 6.2.1. PCR
- 6.2.2. ELISA
- 6.2.3. Enzymatic Methods
- 6.2.4. DNA Staining
- 6.2.5. Other Technologies
- 6.3. Market Analysis, Insights and Forecast - by Application
- 6.3.1. Cell Line Testing
- 6.3.2. Bioproduction Testing
- 6.3.3. Other Applications
- 6.1. Market Analysis, Insights and Forecast - by Product
- 7. Europe Mycoplasma Testing Industry Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Product
- 7.1.1. Instruments
- 7.1.2. Kits and Reagents
- 7.2. Market Analysis, Insights and Forecast - by Technology
- 7.2.1. PCR
- 7.2.2. ELISA
- 7.2.3. Enzymatic Methods
- 7.2.4. DNA Staining
- 7.2.5. Other Technologies
- 7.3. Market Analysis, Insights and Forecast - by Application
- 7.3.1. Cell Line Testing
- 7.3.2. Bioproduction Testing
- 7.3.3. Other Applications
- 7.1. Market Analysis, Insights and Forecast - by Product
- 8. Asia Pacific Mycoplasma Testing Industry Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Product
- 8.1.1. Instruments
- 8.1.2. Kits and Reagents
- 8.2. Market Analysis, Insights and Forecast - by Technology
- 8.2.1. PCR
- 8.2.2. ELISA
- 8.2.3. Enzymatic Methods
- 8.2.4. DNA Staining
- 8.2.5. Other Technologies
- 8.3. Market Analysis, Insights and Forecast - by Application
- 8.3.1. Cell Line Testing
- 8.3.2. Bioproduction Testing
- 8.3.3. Other Applications
- 8.1. Market Analysis, Insights and Forecast - by Product
- 9. Middle East and Africa Mycoplasma Testing Industry Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Product
- 9.1.1. Instruments
- 9.1.2. Kits and Reagents
- 9.2. Market Analysis, Insights and Forecast - by Technology
- 9.2.1. PCR
- 9.2.2. ELISA
- 9.2.3. Enzymatic Methods
- 9.2.4. DNA Staining
- 9.2.5. Other Technologies
- 9.3. Market Analysis, Insights and Forecast - by Application
- 9.3.1. Cell Line Testing
- 9.3.2. Bioproduction Testing
- 9.3.3. Other Applications
- 9.1. Market Analysis, Insights and Forecast - by Product
- 10. South America Mycoplasma Testing Industry Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Product
- 10.1.1. Instruments
- 10.1.2. Kits and Reagents
- 10.2. Market Analysis, Insights and Forecast - by Technology
- 10.2.1. PCR
- 10.2.2. ELISA
- 10.2.3. Enzymatic Methods
- 10.2.4. DNA Staining
- 10.2.5. Other Technologies
- 10.3. Market Analysis, Insights and Forecast - by Application
- 10.3.1. Cell Line Testing
- 10.3.2. Bioproduction Testing
- 10.3.3. Other Applications
- 10.1. Market Analysis, Insights and Forecast - by Product
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 Merck KGaA
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Eurofins Scientific
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Charles River Laboratories International Inc
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Thermo Fisher Scientific Inc
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Bionique Testing Laboratories Inc
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 PromoCell GmbH
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Lonza Group Ltd
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Sartorius AG
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 ATCC
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 AdvaCare
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Agilent Technologies
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.1 Merck KGaA
List of Figures
- Figure 1: Global Mycoplasma Testing Industry Revenue Breakdown (million, %) by Region 2025 & 2033
- Figure 2: Global Mycoplasma Testing Industry Volume Breakdown (K Unit, %) by Region 2025 & 2033
- Figure 3: North America Mycoplasma Testing Industry Revenue (million), by Product 2025 & 2033
- Figure 4: North America Mycoplasma Testing Industry Volume (K Unit), by Product 2025 & 2033
- Figure 5: North America Mycoplasma Testing Industry Revenue Share (%), by Product 2025 & 2033
- Figure 6: North America Mycoplasma Testing Industry Volume Share (%), by Product 2025 & 2033
- Figure 7: North America Mycoplasma Testing Industry Revenue (million), by Technology 2025 & 2033
- Figure 8: North America Mycoplasma Testing Industry Volume (K Unit), by Technology 2025 & 2033
- Figure 9: North America Mycoplasma Testing Industry Revenue Share (%), by Technology 2025 & 2033
- Figure 10: North America Mycoplasma Testing Industry Volume Share (%), by Technology 2025 & 2033
- Figure 11: North America Mycoplasma Testing Industry Revenue (million), by Application 2025 & 2033
- Figure 12: North America Mycoplasma Testing Industry Volume (K Unit), by Application 2025 & 2033
- Figure 13: North America Mycoplasma Testing Industry Revenue Share (%), by Application 2025 & 2033
- Figure 14: North America Mycoplasma Testing Industry Volume Share (%), by Application 2025 & 2033
- Figure 15: North America Mycoplasma Testing Industry Revenue (million), by Country 2025 & 2033
- Figure 16: North America Mycoplasma Testing Industry Volume (K Unit), by Country 2025 & 2033
- Figure 17: North America Mycoplasma Testing Industry Revenue Share (%), by Country 2025 & 2033
- Figure 18: North America Mycoplasma Testing Industry Volume Share (%), by Country 2025 & 2033
- Figure 19: Europe Mycoplasma Testing Industry Revenue (million), by Product 2025 & 2033
- Figure 20: Europe Mycoplasma Testing Industry Volume (K Unit), by Product 2025 & 2033
- Figure 21: Europe Mycoplasma Testing Industry Revenue Share (%), by Product 2025 & 2033
- Figure 22: Europe Mycoplasma Testing Industry Volume Share (%), by Product 2025 & 2033
- Figure 23: Europe Mycoplasma Testing Industry Revenue (million), by Technology 2025 & 2033
- Figure 24: Europe Mycoplasma Testing Industry Volume (K Unit), by Technology 2025 & 2033
- Figure 25: Europe Mycoplasma Testing Industry Revenue Share (%), by Technology 2025 & 2033
- Figure 26: Europe Mycoplasma Testing Industry Volume Share (%), by Technology 2025 & 2033
- Figure 27: Europe Mycoplasma Testing Industry Revenue (million), by Application 2025 & 2033
- Figure 28: Europe Mycoplasma Testing Industry Volume (K Unit), by Application 2025 & 2033
- Figure 29: Europe Mycoplasma Testing Industry Revenue Share (%), by Application 2025 & 2033
- Figure 30: Europe Mycoplasma Testing Industry Volume Share (%), by Application 2025 & 2033
- Figure 31: Europe Mycoplasma Testing Industry Revenue (million), by Country 2025 & 2033
- Figure 32: Europe Mycoplasma Testing Industry Volume (K Unit), by Country 2025 & 2033
- Figure 33: Europe Mycoplasma Testing Industry Revenue Share (%), by Country 2025 & 2033
- Figure 34: Europe Mycoplasma Testing Industry Volume Share (%), by Country 2025 & 2033
- Figure 35: Asia Pacific Mycoplasma Testing Industry Revenue (million), by Product 2025 & 2033
- Figure 36: Asia Pacific Mycoplasma Testing Industry Volume (K Unit), by Product 2025 & 2033
- Figure 37: Asia Pacific Mycoplasma Testing Industry Revenue Share (%), by Product 2025 & 2033
- Figure 38: Asia Pacific Mycoplasma Testing Industry Volume Share (%), by Product 2025 & 2033
- Figure 39: Asia Pacific Mycoplasma Testing Industry Revenue (million), by Technology 2025 & 2033
- Figure 40: Asia Pacific Mycoplasma Testing Industry Volume (K Unit), by Technology 2025 & 2033
- Figure 41: Asia Pacific Mycoplasma Testing Industry Revenue Share (%), by Technology 2025 & 2033
- Figure 42: Asia Pacific Mycoplasma Testing Industry Volume Share (%), by Technology 2025 & 2033
- Figure 43: Asia Pacific Mycoplasma Testing Industry Revenue (million), by Application 2025 & 2033
- Figure 44: Asia Pacific Mycoplasma Testing Industry Volume (K Unit), by Application 2025 & 2033
- Figure 45: Asia Pacific Mycoplasma Testing Industry Revenue Share (%), by Application 2025 & 2033
- Figure 46: Asia Pacific Mycoplasma Testing Industry Volume Share (%), by Application 2025 & 2033
- Figure 47: Asia Pacific Mycoplasma Testing Industry Revenue (million), by Country 2025 & 2033
- Figure 48: Asia Pacific Mycoplasma Testing Industry Volume (K Unit), by Country 2025 & 2033
- Figure 49: Asia Pacific Mycoplasma Testing Industry Revenue Share (%), by Country 2025 & 2033
- Figure 50: Asia Pacific Mycoplasma Testing Industry Volume Share (%), by Country 2025 & 2033
- Figure 51: Middle East and Africa Mycoplasma Testing Industry Revenue (million), by Product 2025 & 2033
- Figure 52: Middle East and Africa Mycoplasma Testing Industry Volume (K Unit), by Product 2025 & 2033
- Figure 53: Middle East and Africa Mycoplasma Testing Industry Revenue Share (%), by Product 2025 & 2033
- Figure 54: Middle East and Africa Mycoplasma Testing Industry Volume Share (%), by Product 2025 & 2033
- Figure 55: Middle East and Africa Mycoplasma Testing Industry Revenue (million), by Technology 2025 & 2033
- Figure 56: Middle East and Africa Mycoplasma Testing Industry Volume (K Unit), by Technology 2025 & 2033
- Figure 57: Middle East and Africa Mycoplasma Testing Industry Revenue Share (%), by Technology 2025 & 2033
- Figure 58: Middle East and Africa Mycoplasma Testing Industry Volume Share (%), by Technology 2025 & 2033
- Figure 59: Middle East and Africa Mycoplasma Testing Industry Revenue (million), by Application 2025 & 2033
- Figure 60: Middle East and Africa Mycoplasma Testing Industry Volume (K Unit), by Application 2025 & 2033
- Figure 61: Middle East and Africa Mycoplasma Testing Industry Revenue Share (%), by Application 2025 & 2033
- Figure 62: Middle East and Africa Mycoplasma Testing Industry Volume Share (%), by Application 2025 & 2033
- Figure 63: Middle East and Africa Mycoplasma Testing Industry Revenue (million), by Country 2025 & 2033
- Figure 64: Middle East and Africa Mycoplasma Testing Industry Volume (K Unit), by Country 2025 & 2033
- Figure 65: Middle East and Africa Mycoplasma Testing Industry Revenue Share (%), by Country 2025 & 2033
- Figure 66: Middle East and Africa Mycoplasma Testing Industry Volume Share (%), by Country 2025 & 2033
- Figure 67: South America Mycoplasma Testing Industry Revenue (million), by Product 2025 & 2033
- Figure 68: South America Mycoplasma Testing Industry Volume (K Unit), by Product 2025 & 2033
- Figure 69: South America Mycoplasma Testing Industry Revenue Share (%), by Product 2025 & 2033
- Figure 70: South America Mycoplasma Testing Industry Volume Share (%), by Product 2025 & 2033
- Figure 71: South America Mycoplasma Testing Industry Revenue (million), by Technology 2025 & 2033
- Figure 72: South America Mycoplasma Testing Industry Volume (K Unit), by Technology 2025 & 2033
- Figure 73: South America Mycoplasma Testing Industry Revenue Share (%), by Technology 2025 & 2033
- Figure 74: South America Mycoplasma Testing Industry Volume Share (%), by Technology 2025 & 2033
- Figure 75: South America Mycoplasma Testing Industry Revenue (million), by Application 2025 & 2033
- Figure 76: South America Mycoplasma Testing Industry Volume (K Unit), by Application 2025 & 2033
- Figure 77: South America Mycoplasma Testing Industry Revenue Share (%), by Application 2025 & 2033
- Figure 78: South America Mycoplasma Testing Industry Volume Share (%), by Application 2025 & 2033
- Figure 79: South America Mycoplasma Testing Industry Revenue (million), by Country 2025 & 2033
- Figure 80: South America Mycoplasma Testing Industry Volume (K Unit), by Country 2025 & 2033
- Figure 81: South America Mycoplasma Testing Industry Revenue Share (%), by Country 2025 & 2033
- Figure 82: South America Mycoplasma Testing Industry Volume Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Mycoplasma Testing Industry Revenue million Forecast, by Product 2020 & 2033
- Table 2: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Product 2020 & 2033
- Table 3: Global Mycoplasma Testing Industry Revenue million Forecast, by Technology 2020 & 2033
- Table 4: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 5: Global Mycoplasma Testing Industry Revenue million Forecast, by Application 2020 & 2033
- Table 6: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 7: Global Mycoplasma Testing Industry Revenue million Forecast, by Region 2020 & 2033
- Table 8: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Region 2020 & 2033
- Table 9: Global Mycoplasma Testing Industry Revenue million Forecast, by Product 2020 & 2033
- Table 10: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Product 2020 & 2033
- Table 11: Global Mycoplasma Testing Industry Revenue million Forecast, by Technology 2020 & 2033
- Table 12: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 13: Global Mycoplasma Testing Industry Revenue million Forecast, by Application 2020 & 2033
- Table 14: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 15: Global Mycoplasma Testing Industry Revenue million Forecast, by Country 2020 & 2033
- Table 16: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 17: United States Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 18: United States Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 19: Canada Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 20: Canada Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 21: Mexico Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 22: Mexico Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 23: Global Mycoplasma Testing Industry Revenue million Forecast, by Product 2020 & 2033
- Table 24: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Product 2020 & 2033
- Table 25: Global Mycoplasma Testing Industry Revenue million Forecast, by Technology 2020 & 2033
- Table 26: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 27: Global Mycoplasma Testing Industry Revenue million Forecast, by Application 2020 & 2033
- Table 28: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 29: Global Mycoplasma Testing Industry Revenue million Forecast, by Country 2020 & 2033
- Table 30: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 31: Germany Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 32: Germany Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 33: United Kingdom Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 34: United Kingdom Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 35: France Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 36: France Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 37: Italy Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 38: Italy Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 39: Spain Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 40: Spain Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 41: Rest of Europe Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 42: Rest of Europe Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 43: Global Mycoplasma Testing Industry Revenue million Forecast, by Product 2020 & 2033
- Table 44: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Product 2020 & 2033
- Table 45: Global Mycoplasma Testing Industry Revenue million Forecast, by Technology 2020 & 2033
- Table 46: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 47: Global Mycoplasma Testing Industry Revenue million Forecast, by Application 2020 & 2033
- Table 48: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 49: Global Mycoplasma Testing Industry Revenue million Forecast, by Country 2020 & 2033
- Table 50: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 51: China Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 52: China Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 53: Japan Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 54: Japan Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 55: India Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 56: India Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 57: Australia Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 58: Australia Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 59: South Korea Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 60: South Korea Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 61: Rest of Asia Pacific Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 62: Rest of Asia Pacific Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 63: Global Mycoplasma Testing Industry Revenue million Forecast, by Product 2020 & 2033
- Table 64: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Product 2020 & 2033
- Table 65: Global Mycoplasma Testing Industry Revenue million Forecast, by Technology 2020 & 2033
- Table 66: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 67: Global Mycoplasma Testing Industry Revenue million Forecast, by Application 2020 & 2033
- Table 68: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 69: Global Mycoplasma Testing Industry Revenue million Forecast, by Country 2020 & 2033
- Table 70: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 71: GCC Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 72: GCC Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 73: South Africa Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 74: South Africa Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 75: Rest of Middle East and Africa Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 76: Rest of Middle East and Africa Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 77: Global Mycoplasma Testing Industry Revenue million Forecast, by Product 2020 & 2033
- Table 78: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Product 2020 & 2033
- Table 79: Global Mycoplasma Testing Industry Revenue million Forecast, by Technology 2020 & 2033
- Table 80: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Technology 2020 & 2033
- Table 81: Global Mycoplasma Testing Industry Revenue million Forecast, by Application 2020 & 2033
- Table 82: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Application 2020 & 2033
- Table 83: Global Mycoplasma Testing Industry Revenue million Forecast, by Country 2020 & 2033
- Table 84: Global Mycoplasma Testing Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 85: Brazil Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 86: Brazil Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 87: Argentina Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 88: Argentina Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 89: Rest of South America Mycoplasma Testing Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 90: Rest of South America Mycoplasma Testing Industry Volume (K Unit) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Mycoplasma Testing Industry?
The projected CAGR is approximately 5.3%.
2. Which companies are prominent players in the Mycoplasma Testing Industry?
Key companies in the market include Merck KGaA, Eurofins Scientific, Charles River Laboratories International Inc, Thermo Fisher Scientific Inc, Bionique Testing Laboratories Inc, PromoCell GmbH, Lonza Group Ltd, Sartorius AG, ATCC, AdvaCare, Agilent Technologies.
3. What are the main segments of the Mycoplasma Testing Industry?
The market segments include Product, Technology, Application.
4. Can you provide details about the market size?
The market size is estimated to be USD 972.3 million as of 2022.
5. What are some drivers contributing to market growth?
Increased Government Initiatives and Funding in Research Activities; Increasing Demand for Fast. Accurate. and Affordable Testing; Increasing Cell Culture Contamination.
6. What are the notable trends driving market growth?
Polymerase Chain Reaction (PCR) is Expected to Hold a Significant Market Share Over the Forecast Period.
7. Are there any restraints impacting market growth?
Long and Laborious Detection Process.
8. Can you provide examples of recent developments in the market?
In May 2022, Abbott received United States Food and Drug Administration clearance for its Alinity m STI Assay. The test simultaneously detects and differentiates four common sexually transmitted infections (STIs), including Mycoplasma genitalium (MG).
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Mycoplasma Testing Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Mycoplasma Testing Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Mycoplasma Testing Industry?
To stay informed about further developments, trends, and reports in the Mycoplasma Testing Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


