Key Insights
The North America kidney cancer therapeutics and diagnostics market is experiencing robust growth, driven by increasing prevalence of kidney cancer, advancements in targeted therapies and immunotherapies, and an expanding diagnostic landscape. The market, valued at approximately $XX million in 2025 (assuming a logical estimation based on the provided CAGR and market size information), is projected to maintain a compound annual growth rate (CAGR) of 5.80% from 2025 to 2033. Key therapeutic classes fueling this growth include targeted therapies like angiogenesis inhibitors and mTOR inhibitors, as well as immunotherapies such as monoclonal antibodies and cytokine immunotherapies (e.g., IL-2). The rising adoption of advanced diagnostic tools, enabling early and accurate detection, further contributes to market expansion. Different kidney cancer types, such as clear cell RCC, papillary RCC, and urothelial carcinoma, represent distinct segments with varying treatment approaches and market sizes. Major pharmaceutical companies like Eisai, Bayer, Novartis, and Amgen are key players, investing heavily in R&D and commercialization efforts to capitalize on this expanding market.
Significant growth drivers include the increasing elderly population, a higher incidence of risk factors such as smoking and obesity, and the ongoing development of innovative therapies showing improved efficacy and reduced side effects. However, the market faces certain restraints, including the high cost of advanced therapies, limited access to innovative treatments in certain regions, and the potential for drug resistance. Despite these challenges, the consistent advancement in research and the promising results of newer treatment modalities suggest a positive outlook for the North America kidney cancer therapeutics and diagnostics market. The market's segmentation across therapeutic classes, cancer types, and components (drugs and diagnostics) offers further opportunities for specialized growth and market penetration. The United States represents the largest segment within North America, reflecting its advanced healthcare infrastructure and high prevalence of kidney cancer.
This comprehensive report provides an in-depth analysis of the North America kidney cancer therapeutics and diagnostics industry, offering invaluable insights for industry professionals, investors, and researchers. With a focus on market dynamics, competitive landscape, and future growth projections (2019-2033, Base Year 2025), this report is an essential resource for navigating this rapidly evolving market.
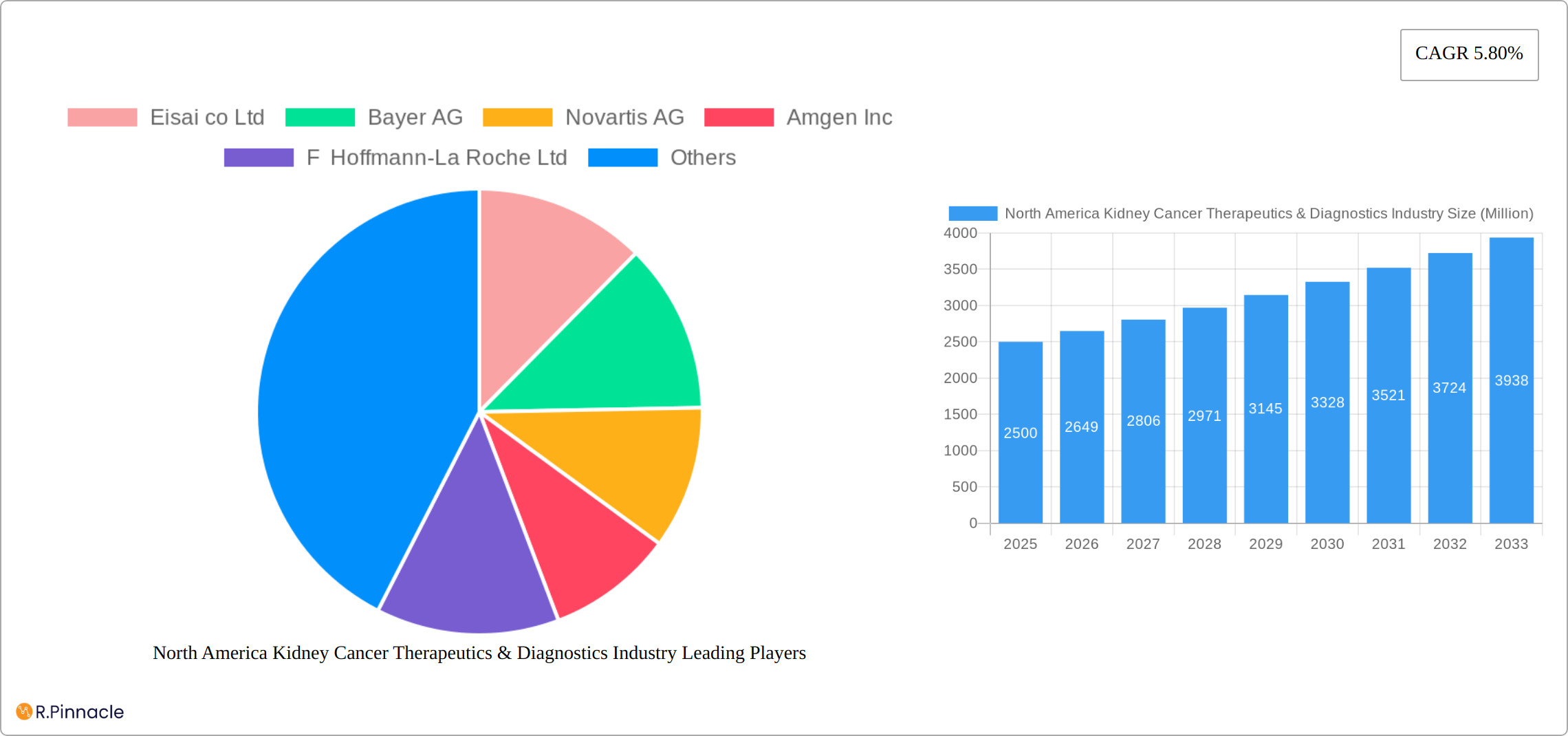
North America Kidney Cancer Therapeutics & Diagnostics Industry Market Structure & Innovation Trends
This section analyzes the market structure, highlighting key players and their market share, and explores innovation drivers shaping the industry's future. The North American kidney cancer therapeutics and diagnostics market is characterized by a moderately concentrated structure with several key players holding significant market share. For example, Eisai co Ltd, Bayer AG, Novartis AG, Amgen Inc, and F Hoffmann-La Roche Ltd collectively command a xx% market share in 2025, based on revenue. The market is driven by factors such as:
- Technological advancements: Development of novel targeted therapies, immunotherapies, and diagnostic tools are driving market growth.
- Rising prevalence of kidney cancer: The increasing incidence of kidney cancer, particularly clear cell renal cell carcinoma (ccRCC), fuels demand for effective treatments.
- Favorable regulatory environment: Regulatory approvals for innovative therapies contribute to market expansion.
- Mergers and Acquisitions (M&A): Significant M&A activities have reshaped the competitive landscape, with deal values exceeding xx Million in the past five years. Examples include [Insert Specific M&A examples if available, otherwise use "several strategic acquisitions focused on expanding therapeutic portfolios and enhancing diagnostic capabilities"].
- Product Substitutes: The emergence of alternative treatment modalities and diagnostic techniques creates both opportunities and competitive pressures.
- End-User Demographics: The aging population and increased awareness of kidney cancer are key factors influencing market demand.

North America Kidney Cancer Therapeutics & Diagnostics Industry Market Dynamics & Trends
The North American kidney cancer therapeutics and diagnostics market is experiencing robust growth, projected to reach xx Million by 2033, exhibiting a CAGR of xx% during the forecast period (2025-2033). This growth is fueled by several key factors:
- Increased adoption of targeted therapies: Targeted therapies, such as angiogenesis inhibitors and mTOR inhibitors, are gaining traction due to their improved efficacy and tolerability profiles.
- Growth of immunotherapy: Immunotherapy, including immune checkpoint inhibitors and cytokine immunotherapy (IL-2), is revolutionizing the treatment landscape, resulting in significant market penetration.
- Advancements in diagnostics: Improved diagnostic techniques, including advanced imaging and molecular diagnostics, enhance early detection and personalized treatment strategies.
- Growing research and development: Continued investments in R&D drive the introduction of novel therapies and diagnostics.
- Shifting consumer preferences: Patients increasingly prefer less invasive and more effective treatment options.
- Competitive dynamics: Intense competition among leading players drives innovation and market expansion.

Dominant Regions & Segments in North America Kidney Cancer Therapeutics & Diagnostics Industry
The US dominates the North American kidney cancer therapeutics and diagnostics market, accounting for the largest market share due to factors such as higher healthcare expenditure, advanced healthcare infrastructure, and higher prevalence rates of kidney cancer. Within the market segments:
- Therapeutic Class: Targeted therapy holds the largest market share followed by immunotherapy. Other therapeutic classes are showing steady growth due to emerging novel treatment approaches.
- Pharmacologic Class: Angiogenesis inhibitors maintain a dominant position due to widespread adoption, with monoclonal antibodies and mTOR inhibitors also experiencing significant growth. Cytokine Immunotherapy (IL-2) holds a niche but stable market share.
- By Cancer Type: Clear cell RCC accounts for the largest share of the market owing to its high prevalence. Papillary RCC and Chromophobe RCC also contribute significantly to market revenue.
- By Component: Drugs segment is the dominant component.
- Key Drivers: Robust healthcare infrastructure, high healthcare expenditure, favorable regulatory environment, and a strong focus on research and development in the US contribute to its market dominance.
North America Kidney Cancer Therapeutics & Diagnostics Industry Product Innovations
Recent years have witnessed significant advancements in kidney cancer therapeutics and diagnostics. Novel targeted therapies with improved efficacy and safety profiles are being introduced. Immunotherapies, such as immune checkpoint inhibitors, have demonstrated remarkable success in extending survival rates. Simultaneously, advanced imaging techniques and molecular diagnostics are improving early detection rates and facilitating personalized treatment strategies, thereby enhancing the overall market outlook. These innovations are not only enhancing treatment outcomes but also driving market expansion.
Report Scope & Segmentation Analysis
This report comprehensively covers the North American kidney cancer therapeutics and diagnostics market, segmented by therapeutic class (Targeted Therapy, Immunotherapy, Other Therapeutic Class), pharmacologic class (Angiogenesis Inhibitors, Monoclonal Antibodies, mTOR Inhibitors, Cytokine Immunotherapy (IL-2)), cancer type (Clear cell RCC, Papillary RCC, Chromophobe RCC, Urothelial carcinoma/Transitional cell carcinoma, Other Kidney cancers), and component (Drugs, Diagnostics). Each segment's growth projections, market sizes, and competitive dynamics are analyzed in detail, providing a granular understanding of the market landscape.
Key Drivers of North America Kidney Cancer Therapeutics & Diagnostics Industry Growth
The growth of the North American kidney cancer therapeutics and diagnostics industry is primarily driven by technological advancements, the rising prevalence of kidney cancer, increased healthcare expenditure, and favorable regulatory frameworks. Specifically, the development of innovative therapies, such as targeted agents and immunotherapies, alongside advancements in diagnostic technologies, significantly contribute to market expansion. Furthermore, government initiatives supporting research and development further fuel this growth trajectory.
Challenges in the North America Kidney Cancer Therapeutics & Diagnostics Industry Sector
Despite the significant growth potential, the industry faces several challenges, including high drug prices, stringent regulatory approvals, potential drug resistance, and the complexities of developing effective immunotherapies. The high cost of treatment poses accessibility challenges. Moreover, the limited success of some therapies in certain kidney cancer subtypes limits market growth for those treatments. Supply chain disruptions caused by geopolitical instability also pose a significant challenge.
Emerging Opportunities in North America Kidney Cancer Therapeutics & Diagnostics Industry
Emerging opportunities lie in the development of personalized medicine approaches, innovative diagnostic tools that allow for earlier and more precise diagnosis, combination therapies leveraging targeted therapy and immunotherapy, and the exploration of novel therapeutic targets. Expansion into underserved markets and improved patient access to treatments also present significant opportunities for growth.
Leading Players in the North America Kidney Cancer Therapeutics & Diagnostics Industry Market
- Eisai co Ltd
- Bayer AG
- Novartis AG
- Amgen Inc
- F Hoffmann-La Roche Ltd
- Abbott Laboratories
- Seattle Genetic
- GlaxoSmithKline PL
- Cerulean Pharma Inc
- Pfizer Inc
Key Developments in North America Kidney Cancer Therapeutics & Diagnostics Industry Industry
- 2023-06: FDA approval of [Insert specific drug approval if available, otherwise replace with “a novel targeted therapy”].
- 2022-11: Launch of a new diagnostic test by [Insert company name if available, otherwise remove bullet point].
- 2021-09: Merger between [Insert company names if available, otherwise remove bullet point].
- [Add more bullet points with specific details as available]
Future Outlook for North America Kidney Cancer Therapeutics & Diagnostics Industry Market
The future of the North American kidney cancer therapeutics and diagnostics market looks promising, driven by continued innovation in treatment modalities and diagnostic capabilities. The focus on personalized medicine, advancements in immunotherapy, and the development of more effective and tolerable therapies will drive significant growth. Strategic partnerships, M&A activity, and increased investment in R&D will further shape the industry's trajectory, presenting substantial opportunities for market expansion and enhanced patient outcomes in the coming decade.
North America Kidney Cancer Therapeutics & Diagnostics Industry Segmentation
-
1. Cancer Type
- 1.1. Clear cell RCC
- 1.2. Papillary RCC
- 1.3. Chromophobe RCC
- 1.4. Urothelial carcinoma/Transitional cell carcinoma
- 1.5. Other Ki
-
2. Component
-
2.1. Drugs
-
2.1.1. Therapeutic Class
- 2.1.1.1. Targeted Therapy
- 2.1.1.2. Immunotherapy
- 2.1.1.3. Other Therapeutic Class
-
2.1.2. Pharmacologic Class
- 2.1.2.1. Angiogenesis Inhibitors
- 2.1.2.2. Monoclonal Antibodies
- 2.1.2.3. mTOR Inhibitors
- 2.1.2.4. Cytokine Immunotherapy (IL-2)
-
2.1.1. Therapeutic Class
-
2.2. Diagnostics
- 2.2.1. Biopsy
- 2.2.2. Imaging Tests
- 2.2.3. Blood Tests
- 2.2.4. Other Diagnostics
-
2.1. Drugs
-
3. Geography
-
3.1. North America
- 3.1.1. United States
- 3.1.2. Canada
- 3.1.3. Mexico
-
3.1. North America
North America Kidney Cancer Therapeutics & Diagnostics Industry Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico

North America Kidney Cancer Therapeutics & Diagnostics Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5.80% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. ; Rising Number of Kidney Cancer Cases; Increased R&D Expenditure of Pharmaceutical Companies
- 3.3. Market Restrains
- 3.3.1. ; High Cost Associated with Treatment; Preference for Generic Drugs
- 3.4. Market Trends
- 3.4.1. Clear cell RCC Segment is Expected to Hold Major Market Share in the North America Kidney Cancer Therapeutics & Diagnostics Market
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. North America Kidney Cancer Therapeutics & Diagnostics Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Cancer Type
- 5.1.1. Clear cell RCC
- 5.1.2. Papillary RCC
- 5.1.3. Chromophobe RCC
- 5.1.4. Urothelial carcinoma/Transitional cell carcinoma
- 5.1.5. Other Ki
- 5.2. Market Analysis, Insights and Forecast - by Component
- 5.2.1. Drugs
- 5.2.1.1. Therapeutic Class
- 5.2.1.1.1. Targeted Therapy
- 5.2.1.1.2. Immunotherapy
- 5.2.1.1.3. Other Therapeutic Class
- 5.2.1.2. Pharmacologic Class
- 5.2.1.2.1. Angiogenesis Inhibitors
- 5.2.1.2.2. Monoclonal Antibodies
- 5.2.1.2.3. mTOR Inhibitors
- 5.2.1.2.4. Cytokine Immunotherapy (IL-2)
- 5.2.1.1. Therapeutic Class
- 5.2.2. Diagnostics
- 5.2.2.1. Biopsy
- 5.2.2.2. Imaging Tests
- 5.2.2.3. Blood Tests
- 5.2.2.4. Other Diagnostics
- 5.2.1. Drugs
- 5.3. Market Analysis, Insights and Forecast - by Geography
- 5.3.1. North America
- 5.3.1.1. United States
- 5.3.1.2. Canada
- 5.3.1.3. Mexico
- 5.3.1. North America
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. North America
- 5.1. Market Analysis, Insights and Forecast - by Cancer Type
- 6. United States North America Kidney Cancer Therapeutics & Diagnostics Industry Analysis, Insights and Forecast, 2019-2031
- 7. Canada North America Kidney Cancer Therapeutics & Diagnostics Industry Analysis, Insights and Forecast, 2019-2031
- 8. Mexico North America Kidney Cancer Therapeutics & Diagnostics Industry Analysis, Insights and Forecast, 2019-2031
- 9. Rest of North America North America Kidney Cancer Therapeutics & Diagnostics Industry Analysis, Insights and Forecast, 2019-2031
- 10. Competitive Analysis
- 10.1. Market Share Analysis 2024
- 10.2. Company Profiles
- 10.2.1 Eisai co Ltd
- 10.2.1.1. Overview
- 10.2.1.2. Products
- 10.2.1.3. SWOT Analysis
- 10.2.1.4. Recent Developments
- 10.2.1.5. Financials (Based on Availability)
- 10.2.2 Bayer AG
- 10.2.2.1. Overview
- 10.2.2.2. Products
- 10.2.2.3. SWOT Analysis
- 10.2.2.4. Recent Developments
- 10.2.2.5. Financials (Based on Availability)
- 10.2.3 Novartis AG
- 10.2.3.1. Overview
- 10.2.3.2. Products
- 10.2.3.3. SWOT Analysis
- 10.2.3.4. Recent Developments
- 10.2.3.5. Financials (Based on Availability)
- 10.2.4 Amgen Inc
- 10.2.4.1. Overview
- 10.2.4.2. Products
- 10.2.4.3. SWOT Analysis
- 10.2.4.4. Recent Developments
- 10.2.4.5. Financials (Based on Availability)
- 10.2.5 F Hoffmann-La Roche Ltd
- 10.2.5.1. Overview
- 10.2.5.2. Products
- 10.2.5.3. SWOT Analysis
- 10.2.5.4. Recent Developments
- 10.2.5.5. Financials (Based on Availability)
- 10.2.6 Abbott Laboratories
- 10.2.6.1. Overview
- 10.2.6.2. Products
- 10.2.6.3. SWOT Analysis
- 10.2.6.4. Recent Developments
- 10.2.6.5. Financials (Based on Availability)
- 10.2.7 Seattle Genetic
- 10.2.7.1. Overview
- 10.2.7.2. Products
- 10.2.7.3. SWOT Analysis
- 10.2.7.4. Recent Developments
- 10.2.7.5. Financials (Based on Availability)
- 10.2.8 GlaxoSmithKline PL
- 10.2.8.1. Overview
- 10.2.8.2. Products
- 10.2.8.3. SWOT Analysis
- 10.2.8.4. Recent Developments
- 10.2.8.5. Financials (Based on Availability)
- 10.2.9 Cerulean Pharma Inc
- 10.2.9.1. Overview
- 10.2.9.2. Products
- 10.2.9.3. SWOT Analysis
- 10.2.9.4. Recent Developments
- 10.2.9.5. Financials (Based on Availability)
- 10.2.10 Pfizer Inc
- 10.2.10.1. Overview
- 10.2.10.2. Products
- 10.2.10.3. SWOT Analysis
- 10.2.10.4. Recent Developments
- 10.2.10.5. Financials (Based on Availability)
- 10.2.1 Eisai co Ltd
List of Figures
- Figure 1: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: North America Kidney Cancer Therapeutics & Diagnostics Industry Share (%) by Company 2024
List of Tables
- Table 1: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: North America Kidney Cancer Therapeutics & Diagnostics Industry Volume K Unit Forecast, by Region 2019 & 2032
- Table 3: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Million Forecast, by Cancer Type 2019 & 2032
- Table 4: North America Kidney Cancer Therapeutics & Diagnostics Industry Volume K Unit Forecast, by Cancer Type 2019 & 2032
- Table 5: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Million Forecast, by Component 2019 & 2032
- Table 6: North America Kidney Cancer Therapeutics & Diagnostics Industry Volume K Unit Forecast, by Component 2019 & 2032
- Table 7: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Million Forecast, by Geography 2019 & 2032
- Table 8: North America Kidney Cancer Therapeutics & Diagnostics Industry Volume K Unit Forecast, by Geography 2019 & 2032
- Table 9: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 10: North America Kidney Cancer Therapeutics & Diagnostics Industry Volume K Unit Forecast, by Region 2019 & 2032
- Table 11: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 12: North America Kidney Cancer Therapeutics & Diagnostics Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 13: United States North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: United States North America Kidney Cancer Therapeutics & Diagnostics Industry Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 15: Canada North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Canada North America Kidney Cancer Therapeutics & Diagnostics Industry Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 17: Mexico North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: Mexico North America Kidney Cancer Therapeutics & Diagnostics Industry Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 19: Rest of North America North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Rest of North America North America Kidney Cancer Therapeutics & Diagnostics Industry Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 21: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Million Forecast, by Cancer Type 2019 & 2032
- Table 22: North America Kidney Cancer Therapeutics & Diagnostics Industry Volume K Unit Forecast, by Cancer Type 2019 & 2032
- Table 23: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Million Forecast, by Component 2019 & 2032
- Table 24: North America Kidney Cancer Therapeutics & Diagnostics Industry Volume K Unit Forecast, by Component 2019 & 2032
- Table 25: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Million Forecast, by Geography 2019 & 2032
- Table 26: North America Kidney Cancer Therapeutics & Diagnostics Industry Volume K Unit Forecast, by Geography 2019 & 2032
- Table 27: North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 28: North America Kidney Cancer Therapeutics & Diagnostics Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 29: United States North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 30: United States North America Kidney Cancer Therapeutics & Diagnostics Industry Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 31: Canada North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 32: Canada North America Kidney Cancer Therapeutics & Diagnostics Industry Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 33: Mexico North America Kidney Cancer Therapeutics & Diagnostics Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 34: Mexico North America Kidney Cancer Therapeutics & Diagnostics Industry Volume (K Unit) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the North America Kidney Cancer Therapeutics & Diagnostics Industry?
The projected CAGR is approximately 5.80%.
2. Which companies are prominent players in the North America Kidney Cancer Therapeutics & Diagnostics Industry?
Key companies in the market include Eisai co Ltd, Bayer AG, Novartis AG, Amgen Inc, F Hoffmann-La Roche Ltd, Abbott Laboratories, Seattle Genetic, GlaxoSmithKline PL, Cerulean Pharma Inc, Pfizer Inc.
3. What are the main segments of the North America Kidney Cancer Therapeutics & Diagnostics Industry?
The market segments include Cancer Type, Component, Geography.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
; Rising Number of Kidney Cancer Cases; Increased R&D Expenditure of Pharmaceutical Companies.
6. What are the notable trends driving market growth?
Clear cell RCC Segment is Expected to Hold Major Market Share in the North America Kidney Cancer Therapeutics & Diagnostics Market.
7. Are there any restraints impacting market growth?
; High Cost Associated with Treatment; Preference for Generic Drugs.
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "North America Kidney Cancer Therapeutics & Diagnostics Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the North America Kidney Cancer Therapeutics & Diagnostics Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the North America Kidney Cancer Therapeutics & Diagnostics Industry?
To stay informed about further developments, trends, and reports in the North America Kidney Cancer Therapeutics & Diagnostics Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



