Key Insights
The global Medical Sterile Packaging Technology market is poised for steady expansion, projected to reach approximately \$38,640 million by 2025, with a Compound Annual Growth Rate (CAGR) of 3.3% anticipated over the forecast period of 2025-2033. This growth is underpinned by a confluence of critical factors, including the escalating demand for sterile medical devices and pharmaceuticals, coupled with increasingly stringent regulatory requirements mandating advanced sterile packaging solutions. The pharmaceutical and biological sector stands out as a significant application segment, driven by the need for enhanced product integrity and patient safety in drug delivery and biopharmaceutical manufacturing. Similarly, the surgical and medical instruments segment is a robust contributor, as advancements in surgical techniques and the proliferation of minimally invasive procedures necessitate specialized sterile packaging that maintains product sterility and facilitates easy access during critical procedures.
Emerging trends in the medical sterile packaging landscape include a pronounced shift towards sustainable and eco-friendly packaging materials, aligning with global environmental concerns and corporate sustainability initiatives. Innovations in material science are leading to the development of advanced polymers, biodegradable alternatives, and smart packaging solutions that offer enhanced barrier properties, tamper-evidence, and traceability. The market also witnesses a growing adoption of sterilization-compatible packaging materials that withstand various sterilization methods such as gamma irradiation, ethylene oxide (EtO), and steam sterilization, without compromising packaging integrity or product efficacy. Geographically, North America and Europe are expected to maintain their dominance due to well-established healthcare infrastructures, high R&D investments, and a strong presence of leading packaging manufacturers. However, the Asia Pacific region is anticipated to exhibit the fastest growth, fueled by rapid industrialization, expanding healthcare access, and a burgeoning medical device manufacturing sector. Key players are actively engaged in strategic collaborations, mergers, and acquisitions to expand their product portfolios, enhance manufacturing capabilities, and strengthen their global footprint in this dynamic market.
Gain unparalleled insights into the global Medical Sterile Packaging Technology market with this comprehensive report. Covering a study period of 2019–2033, with a base year of 2025, this analysis delves deep into market dynamics, key players, and future trends. Essential for industry professionals, manufacturers, and investors, this report provides actionable intelligence to navigate the evolving landscape of sterile medical packaging. The estimated market size is projected to reach several million dollars in the forecast period.
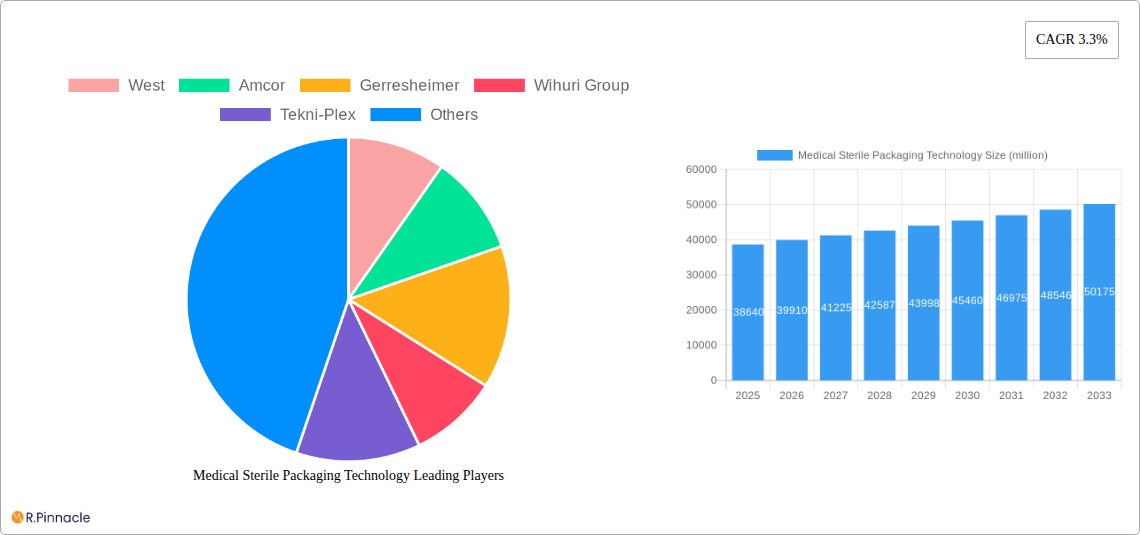
Medical Sterile Packaging Technology Market Structure & Innovation Trends
The Medical Sterile Packaging Technology market exhibits a moderate to high level of concentration, with a few key players like West, Amcor, and Gerresheimer holding significant market share, estimated to be over 60% combined. Innovation is the primary driver of market evolution, fueled by the increasing demand for advanced sterilization methods and improved barrier properties to ensure product integrity. Regulatory frameworks, such as those from the FDA and EMA, play a crucial role in dictating packaging standards and material compliance, thereby shaping technological advancements. While product substitutes exist, they often lack the rigorous validation and performance guarantees of specialized sterile packaging. End-user demographics are dominated by the pharmaceutical and healthcare sectors, with a growing emphasis on biologics and complex medical devices. Mergers and acquisitions (M&A) activities are moderate, with recent deal values in the range of several million dollars, primarily aimed at expanding product portfolios and geographical reach.
- Market Concentration: Moderate to High
- Innovation Drivers: Advanced sterilization techniques, enhanced barrier properties, patient safety concerns.
- Regulatory Frameworks: FDA, EMA, ISO standards.
- Product Substitutes: Limited, often niche applications.
- End-User Demographics: Pharmaceutical, Biotechnology, Medical Device manufacturers.
- M&A Activities: Moderate, strategic acquisitions for portfolio enhancement.
Medical Sterile Packaging Technology Market Dynamics & Trends
The global Medical Sterile Packaging Technology market is poised for robust growth, driven by an escalating demand for safe and effective healthcare solutions. The increasing prevalence of chronic diseases and the continuous development of novel pharmaceutical formulations and advanced medical devices necessitate packaging that guarantees sterility throughout the supply chain. Technological advancements in materials science, such as the development of high-barrier films and antimicrobial coatings, are significantly influencing market trends. These innovations not only enhance product protection but also contribute to extended shelf life, reducing waste and improving cost-efficiency for healthcare providers.
Consumer preferences are increasingly shifting towards single-use, pre-sterilized packaging solutions, particularly for diagnostic kits and surgical instruments, driven by concerns over cross-contamination and infection control. This preference is further amplified by stringent regulatory mandates that prioritize patient safety and compliance. The competitive landscape is characterized by intense innovation and strategic partnerships between packaging manufacturers and medical device/pharmaceutical companies. Companies are investing heavily in research and development to offer customized packaging solutions that meet specific product requirements, including temperature sensitivity, light exposure, and physical stress during transit. The market penetration of advanced sterile packaging technologies is expected to rise significantly as healthcare systems worldwide adopt more sophisticated infection control protocols. The Compound Annual Growth Rate (CAGR) for the Medical Sterile Packaging Technology market is projected to be in the range of xx% for the forecast period.

Dominant Regions & Segments in Medical Sterile Packaging Technology
North America currently leads the Medical Sterile Packaging Technology market, with the United States being the dominant country. This leadership is attributed to its advanced healthcare infrastructure, high spending on medical research and development, and a robust regulatory environment that prioritizes patient safety. The strong presence of major pharmaceutical and medical device manufacturers in the region further fuels demand for sophisticated sterile packaging solutions.
- Leading Region: North America
- Dominant Country: United States
Application Segment Dominance:
The Pharmaceutical and Biological application segment holds the largest market share. This dominance is driven by the continuous development of new drugs, vaccines, and complex biologics that require stringent sterile containment. The increasing focus on personalized medicine and the growing pipeline of biopharmaceuticals are key contributors to this segment's expansion.
- Key Drivers for Pharmaceutical and Biological:
- Rising prevalence of chronic diseases and associated drug demand.
- Growth in biopharmaceutical and vaccine development.
- Stringent regulatory requirements for drug product integrity.
- Demand for sterile packaging for parenteral drugs and biologics.
Type Segment Dominance:
Within the types of sterilization, Physical Sterilization methods, such as gamma irradiation and ethylene oxide (EtO) sterilization, are predominant. These methods are widely accepted for a broad range of medical products due to their efficacy and compatibility with various materials. However, there is a growing trend towards exploring advanced physical sterilization techniques and optimizing chemical sterilization processes to meet evolving material and product needs.
- Key Drivers for Physical Sterilization:
- High efficacy and broad material compatibility.
- Established regulatory acceptance and validation processes.
- Technological advancements in irradiation and steam sterilization.
Medical Sterile Packaging Technology Product Innovations
Recent product innovations in Medical Sterile Packaging Technology focus on enhancing barrier protection, improving sterilization compatibility, and incorporating smart features. Advancements include the development of novel polymer blends offering superior resistance to microbial penetration and chemical degradation, alongside eco-friendlier packaging materials. Innovations in sterilization-compatible films and pouches, such as those resistant to high temperatures or radiation, are gaining traction. The integration of tamper-evident seals and indicators that signal sterilization integrity further enhances product safety and traceability. These developments provide competitive advantages by meeting the increasingly complex needs of pharmaceutical, diagnostic, and surgical instrument manufacturers, ensuring product efficacy and patient safety.
Report Scope & Segmentation Analysis
This report meticulously analyzes the Medical Sterile Packaging Technology market across key segments to provide granular insights.
Application Segments:
- Pharmaceutical and Biological: This segment encompasses packaging solutions for drugs, vaccines, blood products, and other biological materials, projected for substantial growth driven by biopharmaceutical advancements.
- Surgical and Medical Instruments: This segment covers packaging for sterile surgical tools, implants, and various medical devices, experiencing steady growth due to increasing surgical procedures and advancements in medical technology.
- In Vitro Diagnostic Products: This segment includes packaging for diagnostic kits, reagents, and consumables, expected to witness accelerated growth owing to the expanding diagnostics market and the rise of point-of-care testing.
Type Segments:
- Chemical Sterilization: This includes packaging solutions compatible with sterilization methods like ethylene oxide (EtO) and hydrogen peroxide gas, a mature segment with continued demand.
- Physical Sterilization: This segment comprises packaging for gamma irradiation, electron beam, and steam sterilization, demonstrating strong growth driven by the broad applicability of these methods.
Key Drivers of Medical Sterile Packaging Technology Growth
Several factors are propelling the growth of the Medical Sterile Packaging Technology market. The escalating global demand for healthcare services, coupled with the rising incidence of infectious diseases and the aging population, directly translates into a greater need for sterile medical products. Advancements in medical device technology and the development of complex pharmaceutical formulations, including biologics and gene therapies, necessitate packaging solutions that offer superior sterility assurance and protection. Stringent regulatory mandates from global health authorities emphasizing patient safety and infection control also drive the adoption of advanced sterile packaging. Furthermore, continuous innovation in material science and sterilization technologies is creating new opportunities for enhanced product performance and sustainability.
Challenges in the Medical Sterile Packaging Technology Sector
Despite robust growth, the Medical Sterile Packaging Technology sector faces several challenges. The stringent and evolving regulatory landscape requires significant investment in compliance and validation, posing a barrier for smaller players. Supply chain disruptions, material price volatility, and the availability of raw materials can impact production costs and lead times. Increasing demand for sustainable packaging solutions while maintaining sterility and barrier integrity presents a complex technical challenge. Furthermore, intense competition among established players and emerging market entrants can lead to price pressures and necessitate continuous investment in R&D to maintain a competitive edge.
Emerging Opportunities in Medical Sterile Packaging Technology
Emerging opportunities in the Medical Sterile Packaging Technology market are diverse and promising. The burgeoning biopharmaceutical sector, with its specialized requirements for temperature-sensitive and high-value biologics, presents a significant growth avenue. The increasing adoption of advanced sterilization techniques, such as supercritical CO2 sterilization, opens up possibilities for novel packaging materials and designs. The growing trend towards personalized medicine and home healthcare solutions is creating demand for smaller, more convenient, and sterile packaging formats. Furthermore, the integration of smart technologies, including sensors and indicators for real-time monitoring of sterility and environmental conditions, offers opportunities for value-added packaging solutions.
Leading Players in the Medical Sterile Packaging Technology Market
- West
- Amcor
- Gerresheimer
- Wihuri Group
- Tekni-Plex
- Sealed Air
- OLIVER
- ProAmpac
- Printpack
- ALPLA
- Nelipak Healthcare
- VP Group
- OKADA SHIGYO
Key Developments in Medical Sterile Packaging Technology Industry
- 2024 (Ongoing): Increased focus on sustainable sterile packaging materials and reduced environmental impact in production processes.
- 2023: Launch of novel high-barrier films with enhanced protection against moisture and oxygen for sensitive pharmaceuticals.
- 2022: Significant investment in R&D for advanced sterilization techniques and their compatible packaging materials.
- 2021: Strategic partnerships formed between packaging manufacturers and biotechnology firms to develop specialized sterile packaging for advanced therapies.
- 2020: Enhanced adoption of RFID and smart packaging technologies for improved traceability and supply chain management.
- 2019: Introduction of new EtO-compatible packaging solutions meeting evolving regulatory standards.
Future Outlook for Medical Sterile Packaging Technology Market
- 2024 (Ongoing): Increased focus on sustainable sterile packaging materials and reduced environmental impact in production processes.
- 2023: Launch of novel high-barrier films with enhanced protection against moisture and oxygen for sensitive pharmaceuticals.
- 2022: Significant investment in R&D for advanced sterilization techniques and their compatible packaging materials.
- 2021: Strategic partnerships formed between packaging manufacturers and biotechnology firms to develop specialized sterile packaging for advanced therapies.
- 2020: Enhanced adoption of RFID and smart packaging technologies for improved traceability and supply chain management.
- 2019: Introduction of new EtO-compatible packaging solutions meeting evolving regulatory standards.
Future Outlook for Medical Sterile Packaging Technology Market
The future outlook for the Medical Sterile Packaging Technology market is exceptionally positive, driven by the persistent demand for safe and reliable healthcare products. The increasing global healthcare expenditure, coupled with ongoing advancements in medical science and a growing emphasis on infection prevention, will continue to fuel market expansion. Innovations in smart packaging, sustainable materials, and novel sterilization-compatible technologies are expected to create significant growth accelerators. The market is anticipated to witness further consolidation through strategic acquisitions as companies seek to expand their technological capabilities and market reach. The increasing complexity of medical devices and pharmaceuticals will necessitate a parallel evolution in sterile packaging, ensuring continued innovation and market growth for the foreseeable future.
Medical Sterile Packaging Technology Segmentation
-
1. Application
- 1.1. Pharmaceutical and Biological
- 1.2. Surgical and Medical Instruments
- 1.3. In Vitro Diagnostic Products
-
2. Type
- 2.1. Chemical Sterilization
- 2.2. Physical Sterilization
Medical Sterile Packaging Technology Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific

Medical Sterile Packaging Technology REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 3.3% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Medical Sterile Packaging Technology Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Pharmaceutical and Biological
- 5.1.2. Surgical and Medical Instruments
- 5.1.3. In Vitro Diagnostic Products
- 5.2. Market Analysis, Insights and Forecast - by Type
- 5.2.1. Chemical Sterilization
- 5.2.2. Physical Sterilization
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Medical Sterile Packaging Technology Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Pharmaceutical and Biological
- 6.1.2. Surgical and Medical Instruments
- 6.1.3. In Vitro Diagnostic Products
- 6.2. Market Analysis, Insights and Forecast - by Type
- 6.2.1. Chemical Sterilization
- 6.2.2. Physical Sterilization
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Medical Sterile Packaging Technology Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Pharmaceutical and Biological
- 7.1.2. Surgical and Medical Instruments
- 7.1.3. In Vitro Diagnostic Products
- 7.2. Market Analysis, Insights and Forecast - by Type
- 7.2.1. Chemical Sterilization
- 7.2.2. Physical Sterilization
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Medical Sterile Packaging Technology Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Pharmaceutical and Biological
- 8.1.2. Surgical and Medical Instruments
- 8.1.3. In Vitro Diagnostic Products
- 8.2. Market Analysis, Insights and Forecast - by Type
- 8.2.1. Chemical Sterilization
- 8.2.2. Physical Sterilization
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Medical Sterile Packaging Technology Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Pharmaceutical and Biological
- 9.1.2. Surgical and Medical Instruments
- 9.1.3. In Vitro Diagnostic Products
- 9.2. Market Analysis, Insights and Forecast - by Type
- 9.2.1. Chemical Sterilization
- 9.2.2. Physical Sterilization
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Medical Sterile Packaging Technology Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Pharmaceutical and Biological
- 10.1.2. Surgical and Medical Instruments
- 10.1.3. In Vitro Diagnostic Products
- 10.2. Market Analysis, Insights and Forecast - by Type
- 10.2.1. Chemical Sterilization
- 10.2.2. Physical Sterilization
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 West
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Amcor
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Gerresheimer
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Wihuri Group
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Tekni-Plex
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Sealed Air
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 OLIVER
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 ProAmpac
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Printpack
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 ALPLA
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Nelipak Healthcare
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 VP Group
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 OKADA SHIGYO
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.1 West
List of Figures
- Figure 1: Global Medical Sterile Packaging Technology Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Medical Sterile Packaging Technology Revenue (million), by Application 2024 & 2032
- Figure 3: North America Medical Sterile Packaging Technology Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America Medical Sterile Packaging Technology Revenue (million), by Type 2024 & 2032
- Figure 5: North America Medical Sterile Packaging Technology Revenue Share (%), by Type 2024 & 2032
- Figure 6: North America Medical Sterile Packaging Technology Revenue (million), by Country 2024 & 2032
- Figure 7: North America Medical Sterile Packaging Technology Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Medical Sterile Packaging Technology Revenue (million), by Application 2024 & 2032
- Figure 9: South America Medical Sterile Packaging Technology Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America Medical Sterile Packaging Technology Revenue (million), by Type 2024 & 2032
- Figure 11: South America Medical Sterile Packaging Technology Revenue Share (%), by Type 2024 & 2032
- Figure 12: South America Medical Sterile Packaging Technology Revenue (million), by Country 2024 & 2032
- Figure 13: South America Medical Sterile Packaging Technology Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Medical Sterile Packaging Technology Revenue (million), by Application 2024 & 2032
- Figure 15: Europe Medical Sterile Packaging Technology Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe Medical Sterile Packaging Technology Revenue (million), by Type 2024 & 2032
- Figure 17: Europe Medical Sterile Packaging Technology Revenue Share (%), by Type 2024 & 2032
- Figure 18: Europe Medical Sterile Packaging Technology Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Medical Sterile Packaging Technology Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Medical Sterile Packaging Technology Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa Medical Sterile Packaging Technology Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa Medical Sterile Packaging Technology Revenue (million), by Type 2024 & 2032
- Figure 23: Middle East & Africa Medical Sterile Packaging Technology Revenue Share (%), by Type 2024 & 2032
- Figure 24: Middle East & Africa Medical Sterile Packaging Technology Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Medical Sterile Packaging Technology Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Medical Sterile Packaging Technology Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific Medical Sterile Packaging Technology Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific Medical Sterile Packaging Technology Revenue (million), by Type 2024 & 2032
- Figure 29: Asia Pacific Medical Sterile Packaging Technology Revenue Share (%), by Type 2024 & 2032
- Figure 30: Asia Pacific Medical Sterile Packaging Technology Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Medical Sterile Packaging Technology Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Medical Sterile Packaging Technology Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Medical Sterile Packaging Technology Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global Medical Sterile Packaging Technology Revenue million Forecast, by Type 2019 & 2032
- Table 4: Global Medical Sterile Packaging Technology Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Medical Sterile Packaging Technology Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Medical Sterile Packaging Technology Revenue million Forecast, by Type 2019 & 2032
- Table 7: Global Medical Sterile Packaging Technology Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Medical Sterile Packaging Technology Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Medical Sterile Packaging Technology Revenue million Forecast, by Type 2019 & 2032
- Table 13: Global Medical Sterile Packaging Technology Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Medical Sterile Packaging Technology Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global Medical Sterile Packaging Technology Revenue million Forecast, by Type 2019 & 2032
- Table 19: Global Medical Sterile Packaging Technology Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Medical Sterile Packaging Technology Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global Medical Sterile Packaging Technology Revenue million Forecast, by Type 2019 & 2032
- Table 31: Global Medical Sterile Packaging Technology Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Medical Sterile Packaging Technology Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global Medical Sterile Packaging Technology Revenue million Forecast, by Type 2019 & 2032
- Table 40: Global Medical Sterile Packaging Technology Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Medical Sterile Packaging Technology Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Medical Sterile Packaging Technology?
The projected CAGR is approximately 3.3%.
2. Which companies are prominent players in the Medical Sterile Packaging Technology?
Key companies in the market include West, Amcor, Gerresheimer, Wihuri Group, Tekni-Plex, Sealed Air, OLIVER, ProAmpac, Printpack, ALPLA, Nelipak Healthcare, VP Group, OKADA SHIGYO.
3. What are the main segments of the Medical Sterile Packaging Technology?
The market segments include Application, Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 38640 million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 2900.00, USD 4350.00, and USD 5800.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Medical Sterile Packaging Technology," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Medical Sterile Packaging Technology report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Medical Sterile Packaging Technology?
To stay informed about further developments, trends, and reports in the Medical Sterile Packaging Technology, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



