Key Insights
The global specialty biopharmaceutical excipients market is poised for significant expansion, projected to reach an estimated market size of approximately $7,500 million by 2025, with a robust Compound Annual Growth Rate (CAGR) of around 7.5% anticipated throughout the forecast period (2025-2033). This impressive growth is fueled by a confluence of factors, chief among them the escalating demand for advanced drug delivery systems and the increasing complexity of biologic drug formulations. Biologics, with their inherent sensitivity and intricate structures, necessitate specialized excipients to ensure stability, solubility, and effective delivery, driving innovation and market penetration. The expanding pipeline of biologic drugs, coupled with advancements in pharmaceutical research and development, further underpins this upward trajectory. Furthermore, regulatory bodies' increasing emphasis on drug product quality and patient safety is compelling manufacturers to adopt higher-grade excipients, contributing to market value and volume. The rise of novel therapeutic modalities, such as gene and cell therapies, which often require sophisticated formulation strategies, will also play a pivotal role in shaping the market's future.
Key market drivers include the growing prevalence of chronic diseases, necessitating advanced and stable biopharmaceutical treatments, and the continuous innovation in drug formulation technologies. Solubility enhancers and surfactants are expected to witness substantial demand as pharmaceutical companies strive to improve the bioavailability of poorly soluble active pharmaceutical ingredients (APIs) in biopharmaceuticals. The injection medicine segment is anticipated to lead the market, owing to the parenteral administration being the preferred route for most biologics. Geographically, North America and Europe are expected to remain dominant markets, driven by strong R&D investments, established pharmaceutical industries, and a high concentration of biopharmaceutical manufacturers. However, the Asia Pacific region, particularly China and India, presents substantial growth opportunities, attributed to the burgeoning biopharmaceutical manufacturing capabilities, increasing healthcare expenditure, and a growing patient pool. Restraints, such as the high cost of specialized excipients and stringent regulatory hurdles for new product approvals, may temper growth to some extent. Nonetheless, the overarching trend of innovation and the unmet need for effective biopharmaceutical delivery solutions suggest a bright future for the specialty biopharmaceutical excipients market.
Here's the SEO-optimized, reader-centric report description for specialty biopharmaceutical excipients:
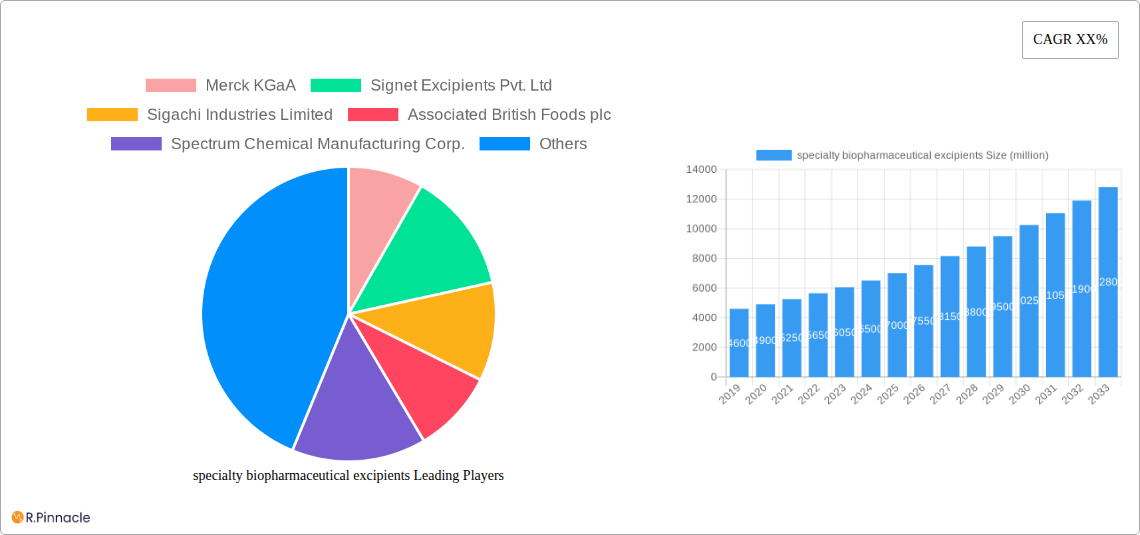
specialty biopharmaceutical excipients Market Structure & Innovation Trends
The global specialty biopharmaceutical excipients market is characterized by a moderate to high level of concentration, with key players investing heavily in research and development to drive innovation. Innovation drivers include the escalating demand for advanced drug delivery systems, the need for improved drug solubility and stability, and the development of novel biologics and complex therapeutics. Regulatory frameworks, such as those established by the FDA and EMA, play a pivotal role in shaping product development and market entry, emphasizing safety, efficacy, and quality. The market also faces the challenge of readily available product substitutes, particularly in the generics space, but the increasing complexity of new drug formulations necessitates specialized excipients with unique functionalities. End-user demographics are shifting towards an aging global population and a rising prevalence of chronic diseases, fueling the demand for specialized pharmaceutical formulations. Mergers and acquisitions (M&A) activities are prevalent, with significant M&A deal values, as larger companies seek to acquire niche technologies and expand their product portfolios. For instance, strategic acquisitions targeting companies with expertise in novel solubility enhancers or sustained-release technologies contribute to market consolidation and the enhancement of competitive advantages. Understanding these structural elements and innovation trends is crucial for stakeholders aiming to navigate this dynamic landscape and capitalize on emerging opportunities.
specialty biopharmaceutical excipients Market Dynamics & Trends
The specialty biopharmaceutical excipients market is poised for robust growth, propelled by a confluence of dynamic factors. A primary growth driver is the escalating global demand for sophisticated pharmaceutical formulations, particularly in areas like targeted drug delivery and biologics, where standard excipients often fall short. The continuous pipeline of new drug approvals, especially for complex molecules requiring specialized handling, directly translates into increased demand for high-performance excipients. Technological disruptions are at the forefront, with advancements in nanotechnology, polymer science, and encapsulation techniques enabling the creation of excipients that offer enhanced drug solubility, bioavailability, and controlled release profiles. This technological evolution is crucial for addressing the bioavailability challenges of many new chemical entities. Consumer preferences are increasingly leaning towards patient-centric drug delivery methods, favoring oral formulations with improved palatability and convenience, as well as injectable medications with enhanced stability and reduced immunogenicity. The competitive dynamics within the market are characterized by intense R&D investments, strategic partnerships between excipient manufacturers and pharmaceutical companies, and a growing emphasis on customized solutions. Companies are differentiating themselves through superior product quality, specialized technical support, and the development of proprietary excipient technologies. The market penetration of advanced excipients is steadily increasing as pharmaceutical companies recognize their critical role in achieving optimal therapeutic outcomes and overcoming formulation hurdles. The projected Compound Annual Growth Rate (CAGR) for this market is strong, reflecting the sustained innovation and increasing adoption of specialty excipients across various therapeutic areas. This dynamic interplay of technological advancement, evolving patient needs, and strategic competition is shaping a vibrant and expanding market.

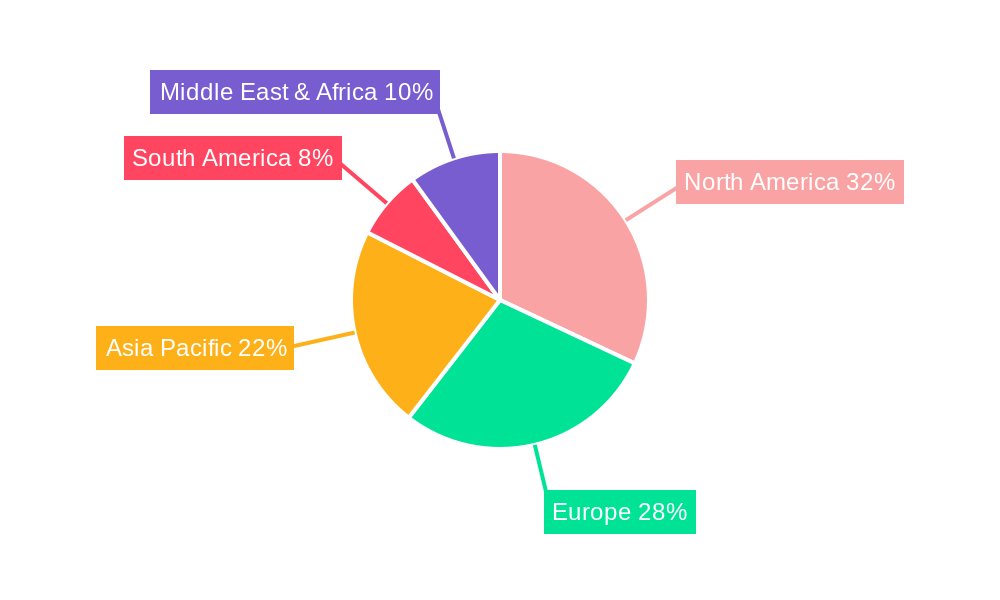
Dominant Regions & Segments in specialty biopharmaceutical excipients
The specialty biopharmaceutical excipients market exhibits distinct regional dominance and segment preferences, driven by economic policies, healthcare infrastructure, and pharmaceutical R&D investments. North America, particularly the United States, stands out as the leading region, owing to its advanced pharmaceutical industry, significant investment in R&D, and a large patient population with a high prevalence of chronic diseases. Robust regulatory frameworks, like those from the FDA, encourage the development and adoption of innovative excipients. Asia-Pacific is emerging as a significant growth engine, fueled by increasing healthcare expenditure, a burgeoning pharmaceutical manufacturing base, and government initiatives promoting domestic drug production and innovation.
Within the Application segment, Oral Medication continues to dominate the market. This is attributed to the widespread patient preference for non-invasive drug administration, the cost-effectiveness of oral dosage forms, and ongoing advancements in oral drug delivery technologies that enhance the efficacy of orally administered drugs. The development of novel excipients enabling better solubility, bioavailability, and sustained release for oral formulations is a key factor. Injection Medicine represents a substantial and growing segment, driven by the increasing development of biologics, vaccines, and injectable formulations for chronic diseases that require precise dosing and controlled release. The demand for high-purity, highly stable injectable excipients is paramount.
In terms of Types, Solubility Enhancers hold a prominent position. A vast number of new chemical entities exhibit poor aqueous solubility, making solubility enhancers indispensable for achieving therapeutic efficacy. These excipients are critical for unlocking the potential of many promising drug candidates. Expanding Agents are also crucial, particularly for solid oral dosage forms, facilitating tablet disintegration and drug dissolution. The ongoing innovation in polymer-based expanding agents contributes to their significant market share. Surfactants play a vital role in various applications, including drug solubilization, emulsification, and improving wetting properties of solid dosage forms, contributing to their widespread use. The continuous development of specialized surfactants tailored for specific drug delivery challenges further solidifies their market presence.
specialty biopharmaceutical excipients Product Innovations
Product innovations in specialty biopharmaceutical excipients are primarily focused on addressing the critical challenges in drug formulation and delivery. Companies are developing advanced excipients that significantly enhance the solubility and bioavailability of poorly soluble drugs, a persistent hurdle in pharmaceutical development. Innovations also include excipients designed for controlled and targeted drug release, enabling precise therapeutic interventions and reducing side effects. The development of novel polymeric excipients for sustained-release formulations and targeted delivery systems, as well as improved surfactants and emulsifiers for complex formulations, are key areas of focus. These advancements offer distinct competitive advantages by enabling pharmaceutical companies to develop more effective, patient-friendly, and commercially viable drug products.
Report Scope & Segmentation Analysis
This report provides a comprehensive analysis of the specialty biopharmaceutical excipients market, segmented by Application and Type.
Application Segmentation:
- Oral Medication: This segment is projected to experience steady growth due to patient preference for convenient administration and ongoing innovations in oral drug delivery systems, including improved bioavailability and controlled-release technologies. Market size is estimated to reach approximately $XX billion by 2033.
- Injection Medicine: This segment is anticipated to witness significant expansion, driven by the rising development of biologics, vaccines, and peptide therapeutics requiring specialized excipients for stability and effective delivery. Market penetration is expected to increase as more complex injectable formulations enter the market.
- Other: This encompasses topical, ophthalmic, and other specialized drug delivery routes, representing a niche but growing segment with specific excipient requirements.
Type Segmentation:
- Expanding Agent: This segment will continue to be a cornerstone for solid oral dosage forms, with innovations focusing on faster disintegration and dissolution profiles.
- Solubility Enhancer: This is a critical growth area, vital for overcoming formulation challenges with new chemical entities (NCEs). Advances in amorphous solid dispersions and nano-suspension technologies will drive demand.
- Surfactant: These versatile excipients are essential for solubilization, emulsification, and wetting across various dosage forms. The development of high-purity, low-toxicity surfactants is a key trend.
- Other: This includes binders, disintegrants, lubricants, coatings, and other specialized functional excipients, each contributing to specific formulation needs.
Key Drivers of specialty biopharmaceutical excipients Growth
The growth of the specialty biopharmaceutical excipients market is propelled by several key factors. The increasing prevalence of chronic diseases globally necessitates the development of more effective and patient-friendly drug formulations, driving demand for advanced excipients. The burgeoning pipeline of new chemical entities (NCEs), many of which exhibit poor solubility, is a significant driver, creating a strong need for solubility enhancers and advanced delivery systems. Technological advancements in pharmaceutical formulation, particularly in areas like nanotechnology and controlled-release technologies, are creating new opportunities for specialized excipients. Furthermore, stringent regulatory requirements for drug safety and efficacy encourage pharmaceutical companies to invest in high-quality, specialized excipients that can ensure product performance and compliance.
Challenges in the specialty biopharmaceutical excipients Sector
Despite the promising growth, the specialty biopharmaceutical excipients sector faces several challenges. Stringent and evolving regulatory hurdles can lead to lengthy approval processes and increased compliance costs for new excipient development. Supply chain disruptions and the need for robust quality control mechanisms can impact the availability and consistency of specialized raw materials. The high cost associated with developing and manufacturing innovative excipients can translate into higher drug development costs for pharmaceutical companies. Intense competition from established players and the emergence of new entrants also exert pressure on pricing and market share.
Emerging Opportunities in specialty biopharmaceutical excipients
Emerging opportunities in the specialty biopharmaceutical excipients market are diverse and promising. The growing focus on biologics and biosimilars presents significant opportunities for excipients that enhance the stability and delivery of these complex molecules. The increasing demand for personalized medicine and advanced drug delivery systems, such as orally disintegrating tablets and inhalable formulations, opens new avenues for specialized excipient development. Expansion into emerging markets with growing healthcare infrastructure and increasing pharmaceutical production offers substantial growth potential. Furthermore, the development of sustainable and bio-based excipients aligns with global environmental trends and consumer preferences.
Leading Players in the specialty biopharmaceutical excipients Market
- Merck KGaA
- Signet Excipients Pvt. Ltd
- Sigachi Industries Limited
- Associated British Foods plc
- Spectrum Chemical Manufacturing Corp.
- Roquette Freres
- IMCD
- Clariant
- DFE Pharma
- Colorcon
- BASF
- Evonik Industries
- J. RETTENMAIER & SOHNE GmbH
Key Developments in specialty biopharmaceutical excipients Industry
- 2023: Launch of novel lipid-based excipients by Evonik Industries to enhance the solubility of challenging drug molecules.
- 2022: BASF announces strategic investment in R&D for biodegradable polymers for advanced drug delivery systems.
- 2022: Roquette Freres acquires a company specializing in high-purity cellulosic derivatives, strengthening its portfolio for oral solid dosage forms.
- 2021: Merck KGaA expands its excipient manufacturing capacity to meet the growing demand for biologics.
- 2021: Sigachi Industries Limited inaugurates a new state-of-the-art facility for the production of microcrystalline cellulose, a key excipient.
- 2020: Colorcon introduces innovative film coating systems designed for improved tablet performance and patient compliance.
- 2019: DFE Pharma partners with a biotech firm to develop excipients for mRNA-based vaccines.
Future Outlook for specialty biopharmaceutical excipients Market
The future outlook for the specialty biopharmaceutical excipients market is exceptionally bright, characterized by sustained innovation and increasing demand. The market will continue to be shaped by the relentless pursuit of improved drug efficacy, safety, and patient convenience. Anticipate significant advancements in excipients designed for complex biologics, gene therapies, and personalized medicines. The trend towards more sophisticated drug delivery systems, such as nanoparticle formulations and long-acting injectables, will drive the development of highly functional and specialized excipients. Furthermore, a growing emphasis on sustainability and green chemistry will encourage the adoption of eco-friendly excipient solutions. Strategic collaborations, mergers, and acquisitions will remain pivotal in consolidating market leadership and fostering innovation, ensuring a dynamic and growth-oriented future for this critical segment of the pharmaceutical industry.
specialty biopharmaceutical excipients Segmentation
-
1. Application
- 1.1. Oral Medication
- 1.2. Injection Medicine
- 1.3. Other
-
2. Types
- 2.1. Expanding Agent
- 2.2. Solubility Enhancer
- 2.3. Surfactant
- 2.4. Other
specialty biopharmaceutical excipients Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
specialty biopharmaceutical excipients REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global specialty biopharmaceutical excipients Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Oral Medication
- 5.1.2. Injection Medicine
- 5.1.3. Other
- 5.2. Market Analysis, Insights and Forecast - by Types
- 5.2.1. Expanding Agent
- 5.2.2. Solubility Enhancer
- 5.2.3. Surfactant
- 5.2.4. Other
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America specialty biopharmaceutical excipients Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Oral Medication
- 6.1.2. Injection Medicine
- 6.1.3. Other
- 6.2. Market Analysis, Insights and Forecast - by Types
- 6.2.1. Expanding Agent
- 6.2.2. Solubility Enhancer
- 6.2.3. Surfactant
- 6.2.4. Other
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America specialty biopharmaceutical excipients Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Oral Medication
- 7.1.2. Injection Medicine
- 7.1.3. Other
- 7.2. Market Analysis, Insights and Forecast - by Types
- 7.2.1. Expanding Agent
- 7.2.2. Solubility Enhancer
- 7.2.3. Surfactant
- 7.2.4. Other
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe specialty biopharmaceutical excipients Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Oral Medication
- 8.1.2. Injection Medicine
- 8.1.3. Other
- 8.2. Market Analysis, Insights and Forecast - by Types
- 8.2.1. Expanding Agent
- 8.2.2. Solubility Enhancer
- 8.2.3. Surfactant
- 8.2.4. Other
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa specialty biopharmaceutical excipients Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Oral Medication
- 9.1.2. Injection Medicine
- 9.1.3. Other
- 9.2. Market Analysis, Insights and Forecast - by Types
- 9.2.1. Expanding Agent
- 9.2.2. Solubility Enhancer
- 9.2.3. Surfactant
- 9.2.4. Other
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific specialty biopharmaceutical excipients Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Oral Medication
- 10.1.2. Injection Medicine
- 10.1.3. Other
- 10.2. Market Analysis, Insights and Forecast - by Types
- 10.2.1. Expanding Agent
- 10.2.2. Solubility Enhancer
- 10.2.3. Surfactant
- 10.2.4. Other
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Merck KGaA
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Signet Excipients Pvt. Ltd
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Sigachi Industries Limited
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Associated British Foods plc
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Spectrum Chemical Manufacturing Corp.
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Roquette Freres
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 IMCD
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Clariant
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 DFE Pharma
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Colorcon
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 BASF
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Evonik Industries
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 J. RETTENMAIER & SOHNE GmbH
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.1 Merck KGaA
List of Figures
- Figure 1: Global specialty biopharmaceutical excipients Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: Global specialty biopharmaceutical excipients Volume Breakdown (K, %) by Region 2024 & 2032
- Figure 3: North America specialty biopharmaceutical excipients Revenue (million), by Application 2024 & 2032
- Figure 4: North America specialty biopharmaceutical excipients Volume (K), by Application 2024 & 2032
- Figure 5: North America specialty biopharmaceutical excipients Revenue Share (%), by Application 2024 & 2032
- Figure 6: North America specialty biopharmaceutical excipients Volume Share (%), by Application 2024 & 2032
- Figure 7: North America specialty biopharmaceutical excipients Revenue (million), by Types 2024 & 2032
- Figure 8: North America specialty biopharmaceutical excipients Volume (K), by Types 2024 & 2032
- Figure 9: North America specialty biopharmaceutical excipients Revenue Share (%), by Types 2024 & 2032
- Figure 10: North America specialty biopharmaceutical excipients Volume Share (%), by Types 2024 & 2032
- Figure 11: North America specialty biopharmaceutical excipients Revenue (million), by Country 2024 & 2032
- Figure 12: North America specialty biopharmaceutical excipients Volume (K), by Country 2024 & 2032
- Figure 13: North America specialty biopharmaceutical excipients Revenue Share (%), by Country 2024 & 2032
- Figure 14: North America specialty biopharmaceutical excipients Volume Share (%), by Country 2024 & 2032
- Figure 15: South America specialty biopharmaceutical excipients Revenue (million), by Application 2024 & 2032
- Figure 16: South America specialty biopharmaceutical excipients Volume (K), by Application 2024 & 2032
- Figure 17: South America specialty biopharmaceutical excipients Revenue Share (%), by Application 2024 & 2032
- Figure 18: South America specialty biopharmaceutical excipients Volume Share (%), by Application 2024 & 2032
- Figure 19: South America specialty biopharmaceutical excipients Revenue (million), by Types 2024 & 2032
- Figure 20: South America specialty biopharmaceutical excipients Volume (K), by Types 2024 & 2032
- Figure 21: South America specialty biopharmaceutical excipients Revenue Share (%), by Types 2024 & 2032
- Figure 22: South America specialty biopharmaceutical excipients Volume Share (%), by Types 2024 & 2032
- Figure 23: South America specialty biopharmaceutical excipients Revenue (million), by Country 2024 & 2032
- Figure 24: South America specialty biopharmaceutical excipients Volume (K), by Country 2024 & 2032
- Figure 25: South America specialty biopharmaceutical excipients Revenue Share (%), by Country 2024 & 2032
- Figure 26: South America specialty biopharmaceutical excipients Volume Share (%), by Country 2024 & 2032
- Figure 27: Europe specialty biopharmaceutical excipients Revenue (million), by Application 2024 & 2032
- Figure 28: Europe specialty biopharmaceutical excipients Volume (K), by Application 2024 & 2032
- Figure 29: Europe specialty biopharmaceutical excipients Revenue Share (%), by Application 2024 & 2032
- Figure 30: Europe specialty biopharmaceutical excipients Volume Share (%), by Application 2024 & 2032
- Figure 31: Europe specialty biopharmaceutical excipients Revenue (million), by Types 2024 & 2032
- Figure 32: Europe specialty biopharmaceutical excipients Volume (K), by Types 2024 & 2032
- Figure 33: Europe specialty biopharmaceutical excipients Revenue Share (%), by Types 2024 & 2032
- Figure 34: Europe specialty biopharmaceutical excipients Volume Share (%), by Types 2024 & 2032
- Figure 35: Europe specialty biopharmaceutical excipients Revenue (million), by Country 2024 & 2032
- Figure 36: Europe specialty biopharmaceutical excipients Volume (K), by Country 2024 & 2032
- Figure 37: Europe specialty biopharmaceutical excipients Revenue Share (%), by Country 2024 & 2032
- Figure 38: Europe specialty biopharmaceutical excipients Volume Share (%), by Country 2024 & 2032
- Figure 39: Middle East & Africa specialty biopharmaceutical excipients Revenue (million), by Application 2024 & 2032
- Figure 40: Middle East & Africa specialty biopharmaceutical excipients Volume (K), by Application 2024 & 2032
- Figure 41: Middle East & Africa specialty biopharmaceutical excipients Revenue Share (%), by Application 2024 & 2032
- Figure 42: Middle East & Africa specialty biopharmaceutical excipients Volume Share (%), by Application 2024 & 2032
- Figure 43: Middle East & Africa specialty biopharmaceutical excipients Revenue (million), by Types 2024 & 2032
- Figure 44: Middle East & Africa specialty biopharmaceutical excipients Volume (K), by Types 2024 & 2032
- Figure 45: Middle East & Africa specialty biopharmaceutical excipients Revenue Share (%), by Types 2024 & 2032
- Figure 46: Middle East & Africa specialty biopharmaceutical excipients Volume Share (%), by Types 2024 & 2032
- Figure 47: Middle East & Africa specialty biopharmaceutical excipients Revenue (million), by Country 2024 & 2032
- Figure 48: Middle East & Africa specialty biopharmaceutical excipients Volume (K), by Country 2024 & 2032
- Figure 49: Middle East & Africa specialty biopharmaceutical excipients Revenue Share (%), by Country 2024 & 2032
- Figure 50: Middle East & Africa specialty biopharmaceutical excipients Volume Share (%), by Country 2024 & 2032
- Figure 51: Asia Pacific specialty biopharmaceutical excipients Revenue (million), by Application 2024 & 2032
- Figure 52: Asia Pacific specialty biopharmaceutical excipients Volume (K), by Application 2024 & 2032
- Figure 53: Asia Pacific specialty biopharmaceutical excipients Revenue Share (%), by Application 2024 & 2032
- Figure 54: Asia Pacific specialty biopharmaceutical excipients Volume Share (%), by Application 2024 & 2032
- Figure 55: Asia Pacific specialty biopharmaceutical excipients Revenue (million), by Types 2024 & 2032
- Figure 56: Asia Pacific specialty biopharmaceutical excipients Volume (K), by Types 2024 & 2032
- Figure 57: Asia Pacific specialty biopharmaceutical excipients Revenue Share (%), by Types 2024 & 2032
- Figure 58: Asia Pacific specialty biopharmaceutical excipients Volume Share (%), by Types 2024 & 2032
- Figure 59: Asia Pacific specialty biopharmaceutical excipients Revenue (million), by Country 2024 & 2032
- Figure 60: Asia Pacific specialty biopharmaceutical excipients Volume (K), by Country 2024 & 2032
- Figure 61: Asia Pacific specialty biopharmaceutical excipients Revenue Share (%), by Country 2024 & 2032
- Figure 62: Asia Pacific specialty biopharmaceutical excipients Volume Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global specialty biopharmaceutical excipients Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global specialty biopharmaceutical excipients Volume K Forecast, by Region 2019 & 2032
- Table 3: Global specialty biopharmaceutical excipients Revenue million Forecast, by Application 2019 & 2032
- Table 4: Global specialty biopharmaceutical excipients Volume K Forecast, by Application 2019 & 2032
- Table 5: Global specialty biopharmaceutical excipients Revenue million Forecast, by Types 2019 & 2032
- Table 6: Global specialty biopharmaceutical excipients Volume K Forecast, by Types 2019 & 2032
- Table 7: Global specialty biopharmaceutical excipients Revenue million Forecast, by Region 2019 & 2032
- Table 8: Global specialty biopharmaceutical excipients Volume K Forecast, by Region 2019 & 2032
- Table 9: Global specialty biopharmaceutical excipients Revenue million Forecast, by Application 2019 & 2032
- Table 10: Global specialty biopharmaceutical excipients Volume K Forecast, by Application 2019 & 2032
- Table 11: Global specialty biopharmaceutical excipients Revenue million Forecast, by Types 2019 & 2032
- Table 12: Global specialty biopharmaceutical excipients Volume K Forecast, by Types 2019 & 2032
- Table 13: Global specialty biopharmaceutical excipients Revenue million Forecast, by Country 2019 & 2032
- Table 14: Global specialty biopharmaceutical excipients Volume K Forecast, by Country 2019 & 2032
- Table 15: United States specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: United States specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 17: Canada specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 18: Canada specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 19: Mexico specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 20: Mexico specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 21: Global specialty biopharmaceutical excipients Revenue million Forecast, by Application 2019 & 2032
- Table 22: Global specialty biopharmaceutical excipients Volume K Forecast, by Application 2019 & 2032
- Table 23: Global specialty biopharmaceutical excipients Revenue million Forecast, by Types 2019 & 2032
- Table 24: Global specialty biopharmaceutical excipients Volume K Forecast, by Types 2019 & 2032
- Table 25: Global specialty biopharmaceutical excipients Revenue million Forecast, by Country 2019 & 2032
- Table 26: Global specialty biopharmaceutical excipients Volume K Forecast, by Country 2019 & 2032
- Table 27: Brazil specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Brazil specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 29: Argentina specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 30: Argentina specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 31: Rest of South America specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 32: Rest of South America specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 33: Global specialty biopharmaceutical excipients Revenue million Forecast, by Application 2019 & 2032
- Table 34: Global specialty biopharmaceutical excipients Volume K Forecast, by Application 2019 & 2032
- Table 35: Global specialty biopharmaceutical excipients Revenue million Forecast, by Types 2019 & 2032
- Table 36: Global specialty biopharmaceutical excipients Volume K Forecast, by Types 2019 & 2032
- Table 37: Global specialty biopharmaceutical excipients Revenue million Forecast, by Country 2019 & 2032
- Table 38: Global specialty biopharmaceutical excipients Volume K Forecast, by Country 2019 & 2032
- Table 39: United Kingdom specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 40: United Kingdom specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 41: Germany specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: Germany specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 43: France specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: France specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 45: Italy specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Italy specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 47: Spain specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 48: Spain specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 49: Russia specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 50: Russia specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 51: Benelux specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 52: Benelux specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 53: Nordics specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 54: Nordics specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 55: Rest of Europe specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 56: Rest of Europe specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 57: Global specialty biopharmaceutical excipients Revenue million Forecast, by Application 2019 & 2032
- Table 58: Global specialty biopharmaceutical excipients Volume K Forecast, by Application 2019 & 2032
- Table 59: Global specialty biopharmaceutical excipients Revenue million Forecast, by Types 2019 & 2032
- Table 60: Global specialty biopharmaceutical excipients Volume K Forecast, by Types 2019 & 2032
- Table 61: Global specialty biopharmaceutical excipients Revenue million Forecast, by Country 2019 & 2032
- Table 62: Global specialty biopharmaceutical excipients Volume K Forecast, by Country 2019 & 2032
- Table 63: Turkey specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 64: Turkey specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 65: Israel specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 66: Israel specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 67: GCC specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 68: GCC specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 69: North Africa specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 70: North Africa specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 71: South Africa specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 72: South Africa specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 73: Rest of Middle East & Africa specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 74: Rest of Middle East & Africa specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 75: Global specialty biopharmaceutical excipients Revenue million Forecast, by Application 2019 & 2032
- Table 76: Global specialty biopharmaceutical excipients Volume K Forecast, by Application 2019 & 2032
- Table 77: Global specialty biopharmaceutical excipients Revenue million Forecast, by Types 2019 & 2032
- Table 78: Global specialty biopharmaceutical excipients Volume K Forecast, by Types 2019 & 2032
- Table 79: Global specialty biopharmaceutical excipients Revenue million Forecast, by Country 2019 & 2032
- Table 80: Global specialty biopharmaceutical excipients Volume K Forecast, by Country 2019 & 2032
- Table 81: China specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 82: China specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 83: India specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 84: India specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 85: Japan specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 86: Japan specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 87: South Korea specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 88: South Korea specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 89: ASEAN specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 90: ASEAN specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 91: Oceania specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 92: Oceania specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
- Table 93: Rest of Asia Pacific specialty biopharmaceutical excipients Revenue (million) Forecast, by Application 2019 & 2032
- Table 94: Rest of Asia Pacific specialty biopharmaceutical excipients Volume (K) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the specialty biopharmaceutical excipients?
The projected CAGR is approximately XX%.
2. Which companies are prominent players in the specialty biopharmaceutical excipients?
Key companies in the market include Merck KGaA, Signet Excipients Pvt. Ltd, Sigachi Industries Limited, Associated British Foods plc, Spectrum Chemical Manufacturing Corp., Roquette Freres, IMCD, Clariant, DFE Pharma, Colorcon, BASF, Evonik Industries, J. RETTENMAIER & SOHNE GmbH.
3. What are the main segments of the specialty biopharmaceutical excipients?
The market segments include Application, Types.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4350.00, USD 6525.00, and USD 8700.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million and volume, measured in K.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "specialty biopharmaceutical excipients," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the specialty biopharmaceutical excipients report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the specialty biopharmaceutical excipients?
To stay informed about further developments, trends, and reports in the specialty biopharmaceutical excipients, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



